- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- 新产品
- 联系我们
Asunaprevir
别名: BMS-650032 中文名称:阿那匹韦
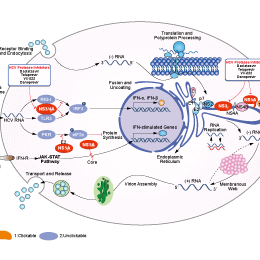
Asunaprevir是具有口服活性的HCV NS3抑制剂。HCV NS3是病毒复制蛋白加工所必需的蛋白酶。
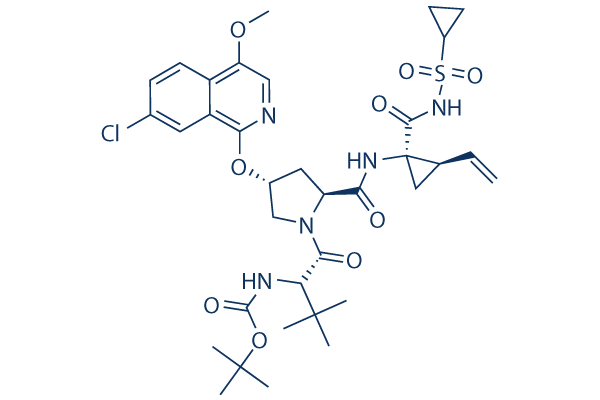
Asunaprevir Chemical Structure
CAS: 630420-16-5


客户使用Selleck的发表文献6篇
产品质控
批次:
纯度:
99.99%
99.99
HCV Protease抑制剂选择性比较
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息 |
|---|---|---|---|---|---|
| Huh7.5 replicon cells | Antiviral assay | 4 days | Antiviral activity against HCV genotype 1b infected in human Huh7.5 replicon cells assessed as reduction in viral replication incubated for 4 days by luciferase reporter gene assay, EC50=0.006μM | 29650290 | |
| HuH7.5 cells | Antiviral assay | 4 days | Antiviral activity against HCV genotype 1b Con1 infected in human HuH7.5 cells assessed as inhibition of viral replication after 4 days by luciferase reporter gene assay, EC50=0.006μM | 29456803 | |
| HuH7 replicon cells | Function assay | 4 days | Inhibition of HCV genotype 1a H77 NS3 protease infected in human HuH7 replicon cells assessed as reduction in viral replication after 4 days by luciferase reporter gene assay, EC50=0.004μM | 29162454 | |
| HuH7 replicon cells | Function assay | 4 days | Inhibition of HCV genotype 1b Con1 NS3 protease infected in human HuH7 replicon cells assessed as reduction in viral replication after 4 days by luciferase reporter gene assay, EC50=0.0012μM | 29162454 | |
| HuH7 cells | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells co-treated with daclatasvir after 3 days by luciferase reporter assay | 28430437 | |
| HuH7 cells | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells at >= 10 times antiviral EC50 after 3 days by luciferase reporter assay | 28430437 | |
| genotype 2a replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 3a S52 NS3 protease in genotype 2a replicon cells by luciferase reporter gene assay, EC50=1.1μM | 27564532 | ||
| genotype 2a replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 2b HC-J8 NS3 protease in genotype 2a replicon cells by luciferase reporter gene assay, EC50=0.621μM | 27564532 | ||
| HCV replicon cells | Antiviral assay | Antiviral activity against HCV genotype 2a JHF-1 in HCV replicon cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.217μM | 27564532 | ||
| HCV replicon cells | Antiviral assay | Antiviral activity against HCV genotype 1a H77 in HCV replicon cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.004μM | 27564532 | ||
| Huh7.5 cells | Antiviral assay | Antiviral activity against HCV genotype 1b Con1 expressing NS3 protease infected in human Huh7.5 cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.003μM | 27564532 | ||
| genotype 1b con1 replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 4a ED43 NS3 protease in genotype 1b con1 replicon cells by luciferase reporter gene assay, EC50=0.0017μM | 27564532 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Asunaprevir是具有口服活性的HCV NS3抑制剂。HCV NS3是病毒复制蛋白加工所必需的蛋白酶。 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 靶点 |
|
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03208322 | Withdrawn | Hepatitis C |
Bristol-Myers Squibb |
November 30 2018 | -- |
| NCT03004625 | Completed | Hepatitis C |
Kaohsiung Medical University Chung-Ho Memorial Hospital|Chang Gung Memorial Hospital|National Taiwan University Hospital|Taipei Veterans General Hospital Taiwan|China Medical University Hospital|National Cheng-Kung University Hospital |
November 2016 | Phase 3 |
| NCT02865369 | Unknown status | Chronic Hepatitis C |
Sang Gyune Kim|Seoul National University Boramae Hospital|Severance Hospital|Inha University Hospital|Korea University|Gachon University Gil Medical Center|Hanyang University Seoul Hospital|Ewha Womans University Mokdong Hospital|Bristol-Myers Squibb|Soonchunhyang University Hospital |
September 2016 | -- |
| NCT02580474 | Completed | Hepatitis C |
Myeong Jun Song|Bristol-Myers Squibb|Soonchunhyang University Hospital|Dankook University|Chungnam National University Hospital|Konyang University Hospital|Eulji University Hospital|Saint Vincent''s Hospital Korea|Konkuk University Hospital|Cheongju St. Mary''s Hospital Cheongju Korea|Severance Hospital|Korea University Guro Hospital|Eulji General Hospital|The Catholic University of Korea |
February 2016 | Phase 4 |
| NCT02496078 | Completed | Hepatitis C |
Bristol-Myers Squibb |
August 2015 | Phase 3 |
| NCT02309450 | Withdrawn | Hepatitis C Virus Genotype 4 Infection |
ANRS Emerging Infectious Diseases|Bristol-Myers Squibb |
December 2014 | Phase 2 |
化学信息&溶解度
| 分子量 | 748.29 | 分子式 | C35H46ClN5O9S |
| CAS号 | 630420-16-5 | SDF | -- |
| Smiles | CC(C)(C)C(C(=O)N1CC(CC1C(=O)NC2(CC2C=C)C(=O)NS(=O)(=O)C3CC3)OC4=NC=C(C5=C4C=C(C=C5)Cl)OC)NC(=O)OC(C)(C)C | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 100 mg/mL ( (133.63 mM); DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 100 mg/mL Water : Insoluble |
摩尔浓度计算器 |
|
体内溶解度 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | ||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项
Tags: buy Asunaprevir | Asunaprevir supplier | purchase Asunaprevir | Asunaprevir cost | Asunaprevir manufacturer | order Asunaprevir | Asunaprevir distributor