
- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Staurosporine (STS)
别名: AM-2282,Antibiotic AM-2282 中文名称:星形孢菌素
Staurosporine (STS)是一种有效的PKC抑制剂,在无细胞试验中作用于PKCα,PKCγ 和PKCη,IC50分别为2 nM,5 nM 和 4 nM,对PKCδ(20 nM)和PKCε(73 nM)作用较弱,对PKCζ (1086 nM)的活性很低。同时 对其他的激酶PKA, PKG, S6K, CaMKII, 等也显示抑制活性。Phase 3。
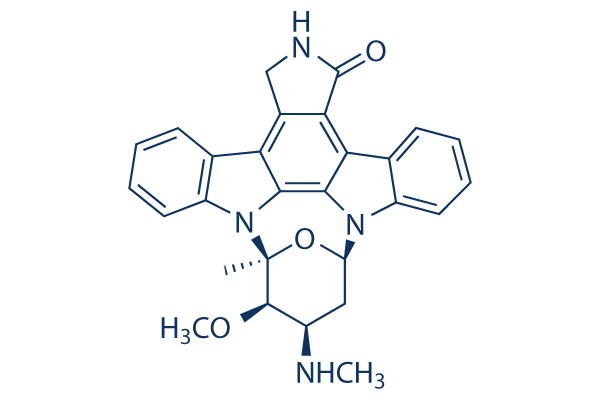
Staurosporine (STS) Chemical Structure
CAS: 62996-74-1


产品质控
批次:
纯度:
99.61%
99.61
常与Staurosporine (STS)一起在实验中被使用的化合物
Retinoic acid (Tretinoin, Vitamin A acid)
Staurosporine和Retinoic acid联用可诱导NB4-R1/NB4-R2细胞的粒细胞分化并抑制其增殖。
Staurosporine (STS)相关产品
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| HepG2 cells | Cytotoxicity assay | 100 uM | 3 days | Cytotoxicity against human HepG2 cells at 100 uM after 3 days by CellTiter-Glo luminescent cell viability assay, IC50 = 0.018 μM. | 28993106 |
| Sf21 cells | Function assay | 30 uM | Inhibition of human CHK2 kinase expressed in insect Sf21 cells using phospho-CREBtide as substrate at 30 uM, IC50 = 0.027 μM. | 21334796 | |
| Sf21 cells | Function assay | 30 uM | Inhibition of human recombinant IKK-alpha expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate at 30 uM, IC50 = 0.039 μM. | 21334796 | |
| Sf21 cells | Function assay | 30 uM | Inhibition of human recombinant IKK-beta expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate at 30 uM, IC50 = 0.49 μM. | 21334796 | |
| A549 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human A549 cells after 72 hrs by sulforhodamine B method, GI50 = 0.0024 μM. | 18484775 | |
| Sf9 cells | Function assay | 20 mins | Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 100 umol/L ATP, IC50 = 0.51 μM. | 17315853 | |
| Sf9 cells | Function assay | 1 min | Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 100 umol/L ATP, IC50 = 0.27 μM. | 17315853 | |
| Sf9 cells | Function assay | 20 mins | Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 10 umol/L ATP, IC50 = 0.083 μM. | 17315853 | |
| Sf9 cells | Function assay | 1 min | Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 10 umol/L ATP, IC50 = 0.036 μM. | 17315853 | |
| Sf9 cells | Function assay | 20 mins | Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 1 umol/L ATP, IC50 = 0.015 μM. | 17315853 | |
| Sf9 cells | Function assay | 1 min | Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 1 umol/L ATP, IC50 = 0.005 μM. | 17315853 | |
| human PBMC | Function assay | 24 h | Suppression of IL2 production in human PBMC after 24 hrs by ELISA, IC50=16 nM. | 18585046 | |
| MDA-MB-231 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by sulforhodamine B method, GI50 = 0.0071 μM. | 18484775 | |
| HT29 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human HT29 cells after 72 hrs by sulforhodamine B method, GI50 = 0.0109 μM. | 18484775 | |
| HT-29 cells | Apoptosis assay | 2 h | Induction of apoptosis in human HT-29 cells assessed as change in mitochondrial transmembrane potential after 2 hrs by JC1 staining based fluorescence assay, ED50 = 0.0026 μM. | 19422206 | |
| HUE cells | Function assay | 90 mins | Inhibition of VEGFR2 in HUE cells assessed as inhibition of VEGF-induced autophosphorylation treated for 90 mins before VEGF challenge by ELISA, IC50 = 0.07 μM. | 20170163 | |
| Sf9 cells | Function assay | 60 mins | Inhibition of human recombinant GST-fused NIK expressed in Sf9 cells after 60 mins by radiometric protein kinase assay, IC50 = 2.3 μM. | 20580552 | |
| HeLa cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 0.000004 μM. | 21388191 | |
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 0.05 μM. | 21388191 | |
| HT-29 cells | Function assay | 2 h | Effect on mitochondrial membrane potential in human HT-29 cells after 2 hrs using JC-1 staining by fluorescence assay, IC50 = 0.002 μM. | 21428375 | |
| HeLa cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 0.000004 μM. | 21488655 | |
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 0.05 μM. | 21488655 | |
| SF268 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human SF268 cells after 48 hrs by SRB assay, GI50 = 0.044 μM. | 21513294 | |
| CHOK1 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human CHOK1 cells after 48 hrs by SRB assay, GI50 = 0.13 μM. | 21513294 | |
| H460 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human H460 cells after 48 hrs by SRB assay, GI50 = 3.6 μM. | 21513294 | |
| HT-29 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HT-29 cells after 48 hrs by SRB assay, GI50 = 3.6 μM. | 21513294 | |
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay, GI50 = 11 μM. | 21513294 | |
| SW1116 cells | Function assay | 24 h | Inhibition of telomerase in human SW1116 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 8.32 μM. | 21962523 | |
| HT-29 cells | Apoptosis assay | 2 h | Induction of apoptosis in human HT-29 cells assessed reduction of mitochondrial membrane potential after 2 hrs by using JC1 staining by fluorescence cell-based assay, ED50 = 0.0026 μM. | 21973101 | |
| LoVo cells | Antiproliferative activity assay | 48 to 72 h | Antiproliferative activity against human LoVo cells after 48 to 72 hrs by MTT assay, IC50 = 0.001 μM. | 22182929 | |
| ST486 cells | Antiproliferative activity assay | 48 to 72 h | Antiproliferative activity against human ST486 cells after 48 to 72 hrs by MTT assay, IC50 = 0.007 μM. | 22182929 | |
| DLD1 cells | Antiproliferative activity assay | 48 to 72 h | Antiproliferative activity against human DLD1 cells after 48 to 72 hrs by MTT assay, IC50 = 0.009 μM. | 22182929 | |
| B16F10 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against mouse B16F10 cells after 48 hrs by MTT assay, IC50 = 2.82 μM. | 22503364 | |
| MCF7 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 3.07 μM. | 22503364 | |
| A431 cells | Cytotoxicity assay | 24 h | Cytotoxicity against human A431 cells after 24 hrs using Annexin VeFITC/propidium iodide staining by MTT assay, IC50 = 0.07 μM. | 22541051 | |
| MCF7 cells | Cytotoxicity assay | 24 h | Cytotoxicity against human MCF7 cells after 24 hrs using Annexin VeFITC/propidium iodide staining by MTT assay, IC50 = 0.18 μM. | 22541051 | |
| BJ cells | Cytotoxicity assay | 72 h | Cytotoxicity against human BJ cells after 72 hrs by Calcein AM assay, IC50 = 0.002 μM. | 22921081 | |
| CEM cells | Cytotoxicity assay | 72 h | Cytotoxicity against human CEM cells after 72 hrs by Calcein AM assay, IC50 = 0.023 μM. | 22921081 | |
| MCF7 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human MCF7 cells after 72 hrs by Calcein AM assay, IC50 = 0.064 μM. | 22921081 | |
| HeLa cells | Cytotoxicity assay | 72 h | Cytotoxicity against human HeLa cells after 72 hrs by Calcein AM assay, IC50 = 0.175 μM. | 22921081 | |
| HepG2 cells | Function assay | 24 h | Inhibition of telomerase in human HepG2 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 4.14 μM. | 23018096 | |
| SW1116 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human SW1116 cells after 48 hrs by MTT method, IC50 = 4.95 μM. | 23018096 | |
| HepG2 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT method, IC50 = 6.73 μM. | 23018096 | |
| BGC823 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human BGC823 cells after 48 hrs by MTT method, IC50 = 6.83 μM. | 23018096 | |
| HeLa cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human HeLa cells after 48 hrs by MTT method, IC50 = 9.12 μM. | 23018096 | |
| HepG2 cells | Function assay | 48 h | Inhibition of telomerase in human HepG2 cells after 48 hrs by TRAP-PCR-ELISA, IC50 = 8.3 μM. | 23279864 | |
| SW1116 cells | Function assay | 24 h | Inhibition of telomerase in human SW1116 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 4.18 μM. | 24286761 | |
| KE-97 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human KE-97 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.13 μM. | 24328283 | |
| Jurkat cells | Cytotoxicity assay | 72 h | Cytotoxicity against human Jurkat cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.14 μM. | 24328283 | |
| HuH7 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human HuH7 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.23 μM. | 24328283 | |
| BGC823 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human BGC823 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.38 μM. | 24328283 | |
| MCF7 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human MCF7 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.52 μM. | 24328283 | |
| NIH/3T3 cells | Cytotoxicity assay | 96 h | Cytotoxicity against mouse NIH/3T3 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| A2780 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A2780 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| HT-29 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human HT-29 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| 8505C cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 8505C cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| 518A2 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 518A2 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| MCF7 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human MCF7 cells after 96 hrs by SRB assay, IC50 = 0.4 μM. | 24361521 | |
| A549 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A549 cells after 96 hrs by SRB assay, IC50 = 0.6 μM. | 24361521 | |
| HEK293 cells | Cytotoxicity assay | 72 h | Cytotoxicity against HEK293 cells after 72 hrs by CellTiterGlo assay, IC50 = 0.056 μM. | 24763262 | |
| HeLa cells | Antiproliferative activity assay | 24 to 48 h | Antiproliferative activity against human HeLa cells after 24 to 48 hrs by CCK-8 assay, IC50 = 2.72 μM. | 24792811 | |
| A549 cells | Antiproliferative activity assay | 24 to 48 h | Antiproliferative activity against human A549 cells after 24 to 48 hrs by CCK-8 assay, IC50 = 3.05 μM. | 24792811 | |
| A549 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 0.02 μM. | 25825934 | |
| HeLa cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 0.025 μM. | 25825934 | |
| PC3 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, IC50 = 0.031 μM. | 25825934 | |
| MGC-803 cells | Function assay | 24 h | Inhibition of telomerase in human MGC-803 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 8.97 μM. | 26900656 | |
| Sf9 insect cells | Function assay | 10 mins | Inhibition of N-terminal GST tagged recombinant full-length human CHK1 expressed in baculovirus Sf9 insect cells after 10 mins by luminescent ADP-Glo assay, IC50 = 0.0012 μM. | 27089211 | |
| Sf21 cells | Function assay | 60 mins | Inhibition of human recombinant JAK2 expressed in Sf21 cells assessed as reduction in Ulight-CAGAGAIETDKEYYTVKD phosphorylation pre-incubated before substrate addition and measured after 60 mins by LANCE detection method, IC50 = 0.002 μM. | 27137359 | |
| Neuro2a cells | Cytotoxicity assay | 72 h | Cytotoxicity against mouse Neuro2a cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 0.0113 μM. | 28152427 | |
| MDA-MB-231 cells | Function assay | 16 h | Inhibition of retinoblastoma protein phosphorylation in human MDA-MB-231 cells after 16 hrs by Hoechst 33342 staining based fluorescence assay, IC50 = 0.066 μM. | 28431342 | |
| Sf9 insect cells | Function assay | 20 mins | Inhibition of recombinant human N-terminally GST-tagged EGFR L858R/T790M/C797S triple mutant expressed in baculovirus in Sf9 insect cells preincubated for 20 mins followed by addition of [33P]-ATP measured after 2 hrs by filter-binding method, IC50 = 0.00029 μM. | 28482151 | |
| HEK293 cells | Cytotoxicity assay | 72 h | Cytotoxicity against HEK293 cells assessed as decrease in cell viability after 72 hrs by CellTiter Glo luminescent assay, CC50 = 0.00354 μM. | 28624701 | |
| BT549 cells | Cytotoxicity assay | 24 h | Cytotoxic activity against human BT549 cells incubated for 24 hrs by MTT assay, IC50 = 0.08 μM. | 28705432 | |
| MDA-MB-468 cells | Cytotoxicity assay | 24 h | Cytotoxic activity against human MDA-MB-468 cells incubated for 24 hrs by MTT assay, IC50 = 0.15 μM. | 28705432 | |
| MDA-MB-231 cells | Cytotoxicity assay | 24 h | Cytotoxic activity against human MDA-MB-231 cells incubated for 24 hrs by MTT assay, IC50 = 0.24 μM. | 28705432 | |
| Vero cells | Cytotoxicity assay | 2 days | Cytotoxicity against African green monkey Vero cells assessed as reduction in cell viability after 2 days by cell-titer-glo luminescent assay, TC50 = 0.0024 μM. | 28763645 | |
| L929 cells | Antiproliferative activity assay | 5 days | Antiproliferative activity against mouse L929 cells after 5 days by WST-1 assay, IC50 = 0.2 μM. | 28956915 | |
| KB-3-1 cells | Antiproliferative activity assay | 5 days | Antiproliferative activity against human KB-3-1 cells after 5 days by WST-1 assay, IC50 = 0.2 μM. | 28956915 | |
| MCF7 cells | Antiproliferative activity assay | 5 days | Antiproliferative activity against human MCF7 cells after 5 days by WST-1 assay, IC50 = 1.8 μM. | 28956915 | |
| FS4-LTM cells | Antiproliferative activity assay | 24 h | Antiproliferative activity against human FS4-LTM cells after 24 hrs by WST-1 assay, IC50 = 2.8 μM. | 28956915 | |
| Vero cells | Cytotoxicity assay | 2 days | Cytotoxicity against African green monkey Vero cells after 2 days by CellTiter-Glo luminescent cell viability assay, IC50 = 0.0024 μM. | 28993106 | |
| NIH/3T3 cells | Cytotoxicity assay | 96 h | Cytotoxicity against mouse NIH/3T3 cells after 96 hrs by SRB assay, EC50 = 0.008 μM. | 29197730 | |
| 518A2 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 518A2 cells after 96 hrs by SRB assay, EC50 = 0.03 μM. | 29197730 | |
| A549 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A549 cells after 96 hrs by SRB assay, EC50 = 0.04 μM. | 29197730 | |
| MCF7 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human MCF7 cells after 96 hrs by SRB assay, EC50 = 0.1 μM. | 29197730 | |
| A2780 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A2780 cells after 96 hrs by SRB assay, EC50 = 0.12 μM. | 29197730 | |
| HT-29 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human HT-29 cells after 96 hrs by SRB assay, EC50 = 0.15 μM. | 29197730 | |
| insect cells | Function assay | 30 mins | Inhibition of recombinant human PDGFRbeta expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 30 mins by LANCE method, IC50 = 0.00076 μM. | 29549836 | |
| Sf9 insect cells | Function assay | 60 mins | Inhibition of recombinant human KDR expressed in Sf9 insect cells using Ulight-CAGAGAIETDKEYYTVKD as substrate after 60 mins by LANCE method, IC50 = 0.0021 μM. | 29549836 | |
| insect cells | Function assay | 10 mins | Inhibition of recombinant human Src expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 10 mins by LANCE method, IC50 = 0.005 μM. | 29549836 | |
| insect cells | Function assay | 15 mins | Inhibition of recombinant human CDK1/Cyclin B expressed in insect cells using Ulight-CFFKNIVTPRTPPPSQGK-amide as substrate after 15 mins by LANCE method, IC50 = 0.025 μM. | 29549836 | |
| insect cells | Function assay | 15 mins | Inhibition of recombinant human CDK1/Cyclin B expressed in insect cells using Ulight-CFFKNIVTPRTPPPSQGK-amide as substrate after 15 mins by LANCE method, IC50 = 0.025 μM. | 29549836 | |
| Sf9 insect cells | Function assay | 15 mins | Inhibition of recombinant human Flt1 expressed in Sf9 insect cells using Ulight-TK peptide as substrate after 15 mins by LANCE method, IC50 = 0.035 μM. | 29549836 | |
| Sf21 insect cells | Function assay | 15 mins | Inhibition of recombinant human Aurora-A expressed in Sf21 insect cells using Ulight-RRRSLLE as substrate after 15 mins by LANCE method, IC50 = 0.039 μM. | 29549836 | |
| insect cells | Function assay | 60 mins | Inhibition of recombinant human AKT1 expressed in insect cells using CREBtide as substrate after 60 mins by LANCE method, IC50 = 0.056 μM. | 29549836 | |
| Sf9 insect cells | Function assay | 60 mins | Inhibition of recombinant human FAK expressed in Sf9 insect cells using Ulight-TK peptide as substrate after 60 mins by LANCE method, IC50 = 0.062 μM. | 29549836 | |
| insect cells | Function assay | 60 mins | Inhibition of recombinant human ABL expressed in insect cells using Ulight-TK peptide as substrate after 60 mins by LANCE method, IC50 = 0.29 μM. | 29549836 | |
| insect cells | Function assay | 60 mins | Inhibition of recombinant human c-MET expressed in insect cells using Ulight-CAGAGAIETDKEYYTVKD as substrate after 60 mins by LANCE method, IC50 = 0.31 μM. | 29549836 | |
| PC3 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human PC3 cells after 72 hrs by SRB assay, IC50 = 0.039 μM. | 29558119 | |
| Sf9 cells | Function assay | 45 mins | Inhibition of recombinant human C-terminal His-tagged/N-terminal GST-tagged EGFR (668 to 1210 residues) expressed in baculovirus infected Sf9 cells using Poly-(Glu, Tyr) as substrate measured after 45 minutes by Kinase-Glo luminescent assay, IC50 = 0.047 μM. | 29902719 | |
| Sf9 cells | Function assay | 45 mins | Inhibition of recombinant human N-terminal GST-tagged HER2 (679 to 1255 residues) expressed in baculovirus infected Sf9 cells using Poly-(Glu, Tyr) as substrate measured after 45 minutes by Kinase-Glo luminescent assay, IC50 = 0.0608 μM. | 29902719 | |
| HT-29 cells | Function assay | 3 h | Inhibition of mitochondrial membrane potential in human HT-29 cells after 3 hrs by JC-1 staining based fluorescence assay, IC50 = 0.02 μM. | 30057155 | |
| HCT116 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HCT116 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 0.03 μM. | 30106291 | |
| Huh7.5 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human Huh7.5 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 0.6 μM. | 30106291 | |
| K562 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human K562 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 1.7 μM. | 30106291 | |
| LO2 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human LO2 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 9.5 μM. | 30106291 | |
| U937 cells | Cytotoxicity assay | Cytotoxicity against human U937 cells, IC50 = 2 μM. | 17088067 | ||
| Sf21 cells | Function assay | Inhibition of JAK3 expressed in Sf21 cells, IC50 = 0.006 μM. | 17088059 | ||
| FL5.12-Akt1 cells | Function assay | Activity against GSK3 phosphorylation in FL5.12-Akt1 cells, EC50 = 0.46 μM. | 16413780 | ||
| MiaPaCa-2 cells | Antiproliferative activity assay | Antiproliferative activity against human MiaPaCa-2 cells, IC50 = 0.37 μM. | 16413780 | ||
| FL5.12-Akt1 cells | Function assay | Activity against GSK3 in FL5.12-Akt1 cells, EC50 = 0.46 μM. | 16403626 | ||
| MiaPaCa-2 cells | Antiproliferative activity assay | Antiproliferative activity against MiaPaCa-2 cells by MTT assay, IC50 = 0.37 μM. | 16403626 | ||
| FL5.12-Akt1 cells | Antiproliferative activity assay | Antiproliferative activity against FL5.12-Akt1 cells by MTT assay, IC50 = 0.29 μM. | 16403626 | ||
| P19 cells | Function assay | Inhibition of Casein kinase 1 in P19 cells, IC50 = 1.4 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Insulin receptor kinase in P19 cells, IC50 = 0.2 μM. | 15771419 | ||
| HEK293 cells | Function assay | Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2, IC50 = 0.077 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Vascular endothelial growth factor receptor in P19 cells, IC50 = 0.014 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Cyclin-dependent kinase 1 in P19 cells, IC50 = 0.008 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Calcium/calmodulin-dependent protein kinase type II in P19 cells, IC50 = 0.006 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Protein Kinase A in P19 cells, IC50 = 0.004 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Platelet-derived growth factor receptor in P19 cells, IC50 = 0.002 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Platelet-derived growth factor receptor in P19 cells, IC50 = 0.002 μM. | 15771419 | ||
| HEK293 cells | Function assay | Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA, IC50 = 0.077 μM. | 12941331 | ||
| DLD-1 cells | Antiproliferative activity assay | Antiproliferative activity against human colon cancer cell line (DLD-1 cells) using MTT assay, IC50 = 0.009 μM. | 11591505 | ||
| ST-486 cells | Antiproliferative activity assay | Antiproliferative activity against burkit lymphoma cell line (ST-486 cells) using MTT assay, IC50 = 0.007 μM. | 11591505 | ||
| umbilical vein endothelial cells | Antiproliferative activity assay | Antiproliferative activity against human umbilical vein endothelial cells (HUVECs) using MTT assay, IC50 = 0.004 μM. | 11591505 | ||
| LoVo cells | Antiproliferative activity assay | Antiproliferative activity against human colon cancer cell line (LoVo cells) using MTT assay, IC50 = 0.001 μM. | 11591505 | ||
| Sf9 insect cells | Function assay | Inhibition of protein kinase C(1:1mixture of PKC alpha & beta) expressed in Sf9 insect cells, IC50 = 0.02 μM. | 9873605 | ||
| endothelial cells | Function assay | Effective dose against plasminogen activator activity stimulated by phorbol ester in endothelial cells, ED50 = 7.5 μM. | 8709095 | ||
| human colon carcinoma cell line HCT116 | Function assay | Concentration required for growth inhibition of human colon carcinoma cell line HCT116, IC50=6 nM. | 15537345 | ||
| MCF7 cells | Function assay | Inhibition of PKA in human MCF7 cells by array-based fluorescence assay, Ki = 0.005 μM. | 18656369 | ||
| MCF7 cells | Function assay | Inhibition of PKA in human MCF7 cells by array-based fluorescence assay, IC50 = 2 μM. | 18656369 | ||
| Sf9 cells | Function assay | Inhibition of human Syk expressed in Sf9 cells, IC50 = 0.003 μM. | 18823784 | ||
| Sf9 cells | Function assay | Inhibition of human ZAP70 expressed in Sf9 cells, IC50 = 0.053 μM. | 18823784 | ||
| insect cells | Function assay | Inhibition of human recombinant Pim1 expressed in insect cells by HTRF, IC50 = 0.01 μM. | 19179076 | ||
| insect Sf21 cells | Function assay | Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation, IC50 = 0.006 μM. | 19427203 | ||
| Jurkat cells | Antiproliferative activity assay | Antiproliferative activity against JAK3 expressing IL2-stimulated human Jurkat cells, IC50 = 0.071 μM. | 19427203 | ||
| Jurkat cells | Antiproliferative activity assay | Antiproliferative activity against JAK3 expressing human resting Jurkat cells, IC50 = 0.311 μM. | 19427203 | ||
| HT-29 cells | Function assay | Inhibition of mitochondrial membrane potential in human HT-29 cells using JC1 dye staining by fluorescence plate reader assay, IC50 = 0.0025 μM. | 21513293 | ||
| HCT116 cells | Function assay | Inhibition of TNIK-mediated TCF4/beta-casein transcription in human HCT116 cells by beta-lactamase reporter gene assay, IC50 = 0.039 μM. | 23232060 | ||
| V79 MZ cells | Function assay | Inhibition of human aldosterone synthase expressed in V79 MZ cells assessed as inhibition of aldosterone synthesis, IC50 = 0.011 μM. | 24422519 | ||
| HepG2 cells | Cytotoxicity assay | Cytotoxicity against human HepG2 cells, EC50 = 2 μM. | 25316317 | ||
| Raji cells | Cytotoxicity assay | Cytotoxicity against human Raji cells, EC50 = 2 μM. | 25316317 | ||
| BJ cells | Cytotoxicity assay | Cytotoxicity against human BJ cells, EC50 = 2 μM. | 25316317 | ||
| HEK293 cells | Cytotoxicity assay | Cytotoxicity against HEK293 cells, EC50 = 2 μM. | 25316317 | ||
| PC3 cells | Cytotoxicity assay | Cytotoxicity against human PC3 cells by sulforhodamine B assay, IC50 = 0.017 μM. | 29389122 | ||
| HCT116 cells | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by sulforhodamine B assay, IC50 = 0.055 μM. | 29389122 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.053 μM. | 30067358 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.053 μM. | 30067358 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.057 μM. | 30067358 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.057 μM. | 30067358 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Staurosporine (STS)是一种有效的PKC抑制剂,在无细胞试验中作用于PKCα,PKCγ 和PKCη,IC50分别为2 nM,5 nM 和 4 nM,对PKCδ(20 nM)和PKCε(73 nM)作用较弱,对PKCζ (1086 nM)的活性很低。同时 对其他的激酶PKA, PKG, S6K, CaMKII, 等也显示抑制活性。Phase 3。 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | Staurosporine 也强抑制HeLa S3细胞,IC50为4 nM。Staurosporine 也抑制多种其他蛋白激酶,包括PKA, PKG, 磷酸激酶, S6 激酶, 肌球蛋白轻链激酶(MLCK), CAM PKII, cdc2, v-Src, Lyn, c-Fgr, 和Syk , IC50分别为 15 nM, 18 nM, 3 nM, 5 nM, 21 nM, 20 nM, 9 nM, 6 nM, 20 nM, 2 nM, 和 16 nM。 Staurosporine (1 μM) 诱导 PC12细胞90%以上凋亡,这种作用可被过量表达的Bcl-2抑制,或者zVAD-fmk, cycloheximide (10 μM) 及actinomycin D (5 μM)处理也可抑制。相应地, Staurosporine处理,诱导细胞内游离钙水平 [Ca2+]i快速且持久的提升,线粒体活性氧(ROS)的积累,及 随后的线粒体功能障碍。通过caspase-8激活和Bid 分裂,功能性caspase-3的表达可增强Staurosporine诱导MCF7细胞死亡。 1 μM Staurosporine处理,只部分抑制IL-3刺激的Bcl2 磷酸化,而完全阻断PKC调节的 Bcl2磷酸化。 Staurosporine 诱导人类包皮成纤维细胞AG-1518凋亡,根据溶酶体组织蛋白酶D调节的 细胞色素 c释放和 caspase 激活。 除了激活传统线粒体凋亡通路, Staurosporine触发一种新型内在凋亡通路, 依赖Apaf-1存在时对caspase-9的激活。 | |||
|---|---|---|---|---|
| 细胞实验 | 细胞系 | PC12 | ||
| 浓度 | 溶于DMSO,终浓度为1 μM | |||
| 孵育时间 | ~32 小时 | |||
| 方法 | 使用Staurosporine处理细胞32小时。细胞在4%多聚甲醛中混合,然后使用DNA结合染料Hoechst 33342染色。在荧光照明下观察细胞,测定凋亡细胞百分数。 | |||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | p-Akt / Akt / PARP /Cleaved PARP FAK / RIP Ambra1 |
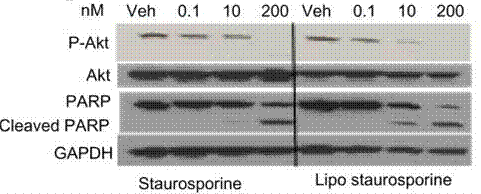
|
24174874 | |
| Growth inhibition assay | Cell viability |

|
25215174 | |
| Immunofluorescence | Tubulin / Actin Phalloidin / Type II collagen Pyk2 Annexin cleaved-caspase 3 FAK 4.47 |
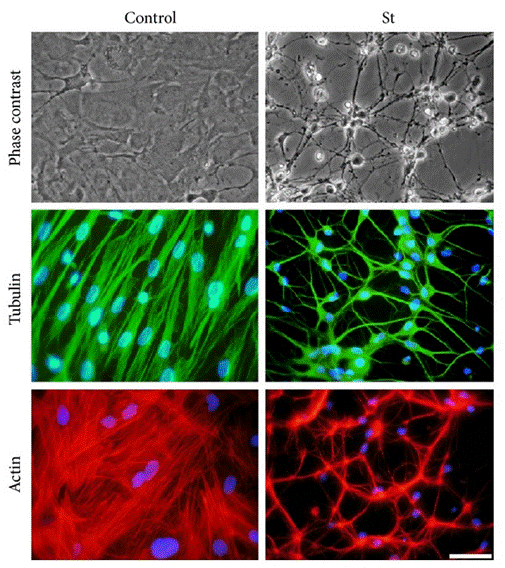
|
25215174 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | 在局部贫血前,使用Staurosporine(0.1-10 ng)预处理局部贫血沙鼠和大鼠模型,可防止神经元损伤,这种作用存在剂量依赖性,说明CAl锥体细胞死亡涉及PKC。 | |
|---|---|---|
| 动物实验 | Animal Models | 短暂局部贫血的雄性Mongolian沙鼠或雄性 Wistar大鼠 |
| Dosages | ~10 ng | |
| Administration | 定向处理到海马双边CA1子域 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05209347 | Withdrawn | Ankle Fractures|Post-traumatic Osteoarthritis|Osteoarthritis Ankle |
University of Iowa |
December 2024 | Not Applicable |
| NCT05956990 | Not yet recruiting | Colorectal Cancer Survivor|Rehabilitation|Quality of Life |
Qu Shen|The First Affiliated Hospital of Xiamen University|Xiamen University |
July 1 2024 | Not Applicable |
| NCT05957289 | Not yet recruiting | Rehabilitation|Colorectal Cancer Survivor|Quality of Life|Pilot Study |
Qu Shen|The First Affiliated Hospital of Xiamen University|Xiamen University |
June 1 2024 | Not Applicable |
| NCT06245135 | Not yet recruiting | Mobility Limitation |
University of Toronto|University Health Network Toronto|March of Dimes Canada|Heart and Stroke Foundation of Canada|University of Alberta|University of Manitoba|Bruyere Research Institute |
June 2024 | Not Applicable |
| NCT06374108 | Not yet recruiting | Multiple Sclerosis |
University of Aarhus|Aarhus University Hospital|University of Copenhagen |
May 1 2024 | Not Applicable |
|
化学信息&溶解度
| 分子量 | 466.53 | 分子式 | C28H26N4O3 |
| CAS号 | 62996-74-1 | SDF | Download Staurosporine (STS) SDF |
| Smiles | CC12C(C(CC(O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)NC)OC | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 46 mg/mL ( (98.6 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项



