- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
(-)-Blebbistatin
别名: (S)-(-)-Blebbistatin 中文名称:布雷他汀
此产品请避光密封保存。
(-)-Blebbistatin ((S)-(-)-Blebbistatin)是一种细胞渗透性抑制剂,作用于非肌肉肌球蛋白IIATPase,无细胞试验中IC50为~2 μM,不抑制肌球蛋白轻链激酶 (MLCK),抑制卵裂沟的缢缩,不干扰有丝分裂或收缩环的组装。
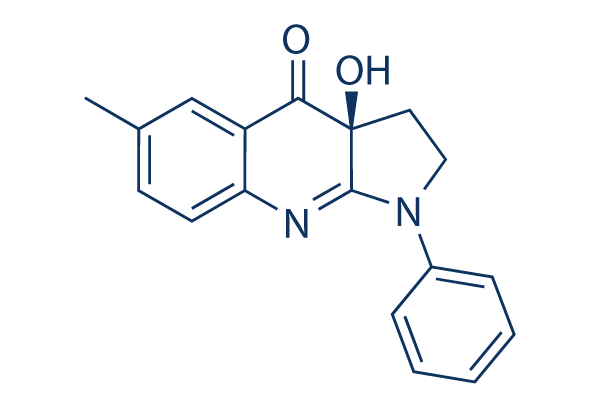
(-)-Blebbistatin Chemical Structure
CAS: 856925-71-8


产品质控
批次:
纯度:
99.82%
99.82
(-)-Blebbistatin相关产品
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | ||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | ||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | (-)-Blebbistatin ((S)-(-)-Blebbistatin)是一种细胞渗透性抑制剂,作用于非肌肉肌球蛋白IIATPase,无细胞试验中IC50为~2 μM,不抑制肌球蛋白轻链激酶 (MLCK),抑制卵裂沟的缢缩,不干扰有丝分裂或收缩环的组装。 | ||
|---|---|---|---|
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | Blebbistatin抑制细胞分裂,改变鱼角膜细胞的平滑运动,且抑制缺乏细丝蛋白的细胞系进行自发起泡。Blebbistatin抑制非肌肉肌球蛋白IIA,非肌肉肌球蛋白IIB,和兔骨骼肌肌球蛋白S1的HMM片段的酶活性,而不抑制平滑肌球蛋白。 Blebbistatin快速且可逆抑制Mg-ATPase活性,也抑制非肌肉肌球蛋白IIA和IIB的体外活性,而极其微弱抑制平滑肌肌球蛋白(IC50=80 μM)。Blebbistatin有效抑制Dictyostelium肌球蛋白II,但极其微弱抑制Acanthamoeba肌球蛋白II。Blebbistatin不抑制代表性的I,V,和X型肌球蛋白超家族成员。 Blebbistatin不与核苷酸竞争性结合到骨骼肌肌球蛋白亚片段-1。Blebbistatin优先结合到ATP酶,与ADP和磷酸盐在活性位点相互作用,减慢磷释放。Blebbistatin既不干扰肌球蛋白与肌动蛋白结合,也不干扰ATP诱导的肌动球蛋白解离。 |
|||
|---|---|---|---|---|
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | talin 1 / vinculin / paxillin paxillin / pY31 paxillin / pY397FAK / FAK PY epitopes / vinculin / paxillin |

|
20308429 | |
| Growth inhibition assay | Cell viability Cell death |
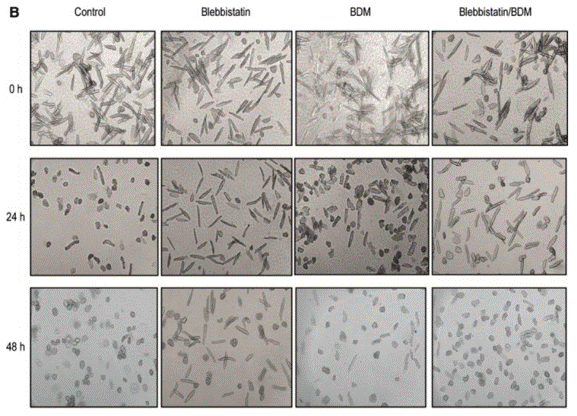
|
26733241 | |
| Glycerol/urea gel electrophoresis | RLC phosphorylation RLC phosphorylation RLC phosphorylation |
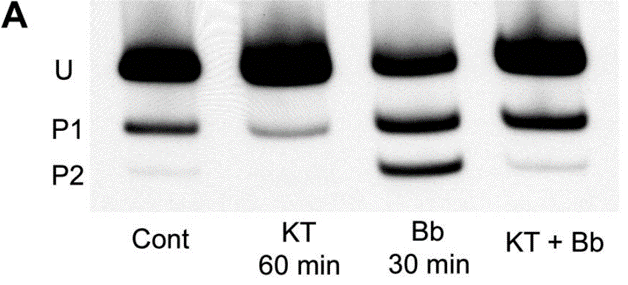
|
18701651 | |
| DIC image | Traction force of a palladin KD (Palld4) cell |
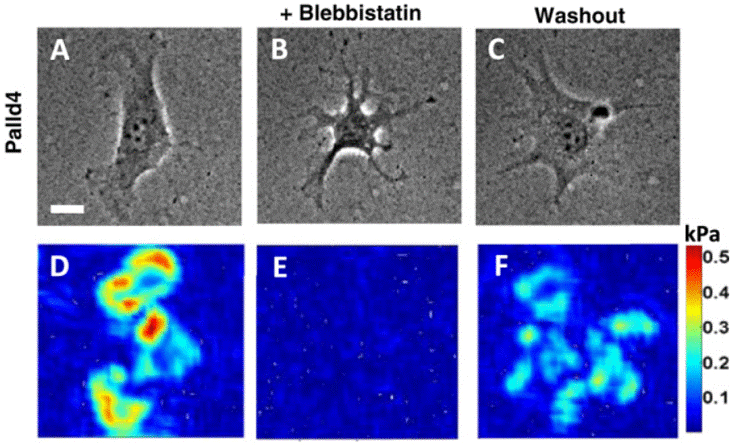
|
27353427 | |
| Immunofluorescence | GCs morphology actin / NMIIA / tubulin VE-cadherin / F-actin PY epitopes / actin PY epitopes / paxillin talin 1 / FAK / β1 integrin / zyxin / vinculin / α-actinin / paxillin |
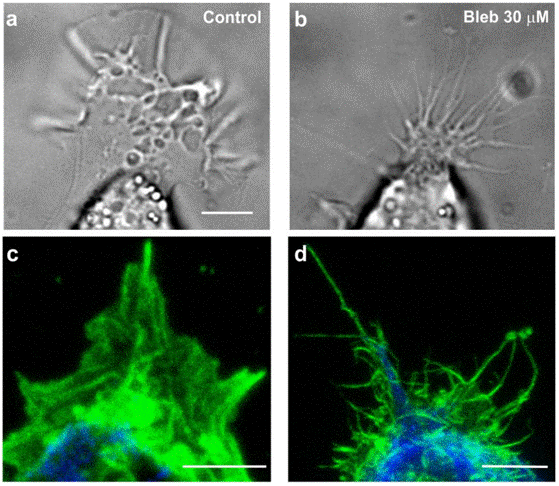
|
25598228 | |
|
化学信息&溶解度
| 分子量 | 292.33 | 分子式 | C18H16N2O2 |
| CAS号 | 856925-71-8 | SDF | Download (-)-Blebbistatin SDF |
| Smiles | CC1=CC2=C(C=C1)N=C3C(C2=O)(CCN3C4=CC=CC=C4)O | ||
| 储存条件(自收到货起) | 3年 -20°C(避光) 粉状 | ||
|
体外溶解度 |
DMSO : 39 mg/mL ( (133.41 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项