
- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Rigosertib (ON-01910)
Rigosertib (ON-01910) 是一种非ATP竞争性PLK1抑制剂,无细胞试验中IC50为9 nM,比作用于Plk2选择性高30倍,对Plk3没有抑制活性。Rigosertib 可抑制 PI3K/Akt 信号通路并激活氧化应激信号。Rigosertib 可诱导多种癌细胞的凋亡。Phase 3。
Rigosertib (ON-01910) Chemical Structure
CAS: 1225497-78-8


产品质控
批次:
纯度:
99.28%
99.28
Rigosertib (ON-01910)相关产品
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| A2780 | Function assay | 0.25 uM | 24 hrs | Reduction in CDC25C level in human A2780 cells at 0.25 uM after 24 hrs by Western blot analysis | 24471873 |
| A2780 | Apoptosis assay | 0.25 uM | 24 hrs | Induction of apoptosis in human A2780 cells assessed as caspase-3/7 activation at 0.25 uM after 24 hrs by using Apo-ONE homogeneous caspase-3/7 kit | 24471873 |
| A2780 | Function assay | 0.25 uM | 24 hrs | Reduction in Mcl1 level in human A2780 cells at 0.25 uM after 24 hrs by Western blot analysis | 24471873 |
| MRC5 | Function assay | 1 uM | Metabolic stability of the compound in human MRC5 cells at 1 uM | 21463944 | |
| MCF7 | Function assay | 1 uM | Metabolic stability of the compound in human MCF7 cells at 1 uM | 21463944 | |
| LNCAP | Antiproliferative assay | 72 hrs | Antiproliferative activity against AR positive human LNCAP cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.025 μM. | 24471873 | |
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.012 μM. | 24471873 | |
| DU145 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human DU145 cells after 96 hrs by trypan blue exclusion assay, IC50 = 0.075 μM. | 21812421 | |
| K562 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human K562 cells after 96 hrs by trypan blue exclusion assay, IC50 = 0.0075 μM. | 21812421 | |
| MRC5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MRC5 cells after 72 hrs by MTT assay, GI50 = 0.71 μM. | 21463944 | |
| MDA468 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MDA468 cells after 24 hrs by MTT assay, GI50 = 0.601 μM. | 21463944 | |
| MDA468 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA468 cells after 48 hrs by MTT assay, GI50 = 0.302 μM. | 21463944 | |
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, GI50 = 0.05 μM. | 21463944 | |
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, GI50 = 0.05 μM. | 21463944 | |
| MDA468 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA468 cells after 72 hrs by MTT assay, GI50 = 0.02 μM. | 21463944 | |
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells after 72 hrs by MTT assay, GI50 = 0.01 μM. | 21463944 | |
| PANC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PANC1 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.039 μM. | 24471873 | |
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against ER positive human MCF7 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.05 μM. | 24471873 | |
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.05 μM. | 24471873 | |
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against ER negative human MDA-MB-231 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.057 μM. | 24471873 | |
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.062 μM. | 24471873 | |
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.07 μM. | 24471873 | |
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against AR negative human DU145 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.075 μM. | 24471873 | |
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Rigosertib (ON-01910) 是一种非ATP竞争性PLK1抑制剂,无细胞试验中IC50为9 nM,比作用于Plk2选择性高30倍,对Plk3没有抑制活性。Rigosertib 可抑制 PI3K/Akt 信号通路并激活氧化应激信号。Rigosertib 可诱导多种癌细胞的凋亡。Phase 3。 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 特性 | Rigosertib是作用于Polo样激酶(PLK1)的非ATP竞争性抑制剂。 | |||||||||||
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | Rigosertib是PLK1的非ATP竞争性抑制剂,IC50为9 nM。Rigosertib也抑制 PLK2,PDGFR,Flt1,BCR-ABL,Fyn,Src和CDK1, IC50为18-260 nM。Rigosertib具有使细胞死亡的活性,作用于94种不同肿瘤细胞系,IC50为50-250 nM,包括BT27,MCF-7,DU145,PC3,U87,A549,H187,RF1,HCT15,SW480,和KB细胞。Rigosertib作用于正常细胞,如 HFL,PrEC,HMEC,和HUVEC没有效果,除非作用浓度高于5-10 µM。100-250 nM Rigosertib作用于HeLa 细胞,诱导纺锤体变异和凋亡。 Rigosertib也抑制一些多重耐药的的肿瘤细胞系,包括 MES-SA, MES-SA/DX5a, CEM, 和 CEM/C2a, IC50为50-100 nM。0.25-5 µM Rigosertib作用于DU145细胞, 抑制细胞周期,使细胞停在G2/M 期,和激活凋亡通路。50 nM-0.5 µM Rigosertib作用于A549细胞,诱导存活力和caspase 3/7激活的丧失。最新研究显示, Rigosertib作用于慢性淋巴细胞性白血病 (CLL)细胞,诱导凋亡,且作用于T细胞或正常B细胞没有毒性。Rigosertib 慢性淋巴细胞性白血病(CLL)细胞,也抑制滤泡树突状细胞的促生存效果,作用于白血病细胞,降低SDF-1诱导的迁移 。 | |||
|---|---|---|---|---|
| 激酶实验 | 体外测定PLK1的酶实验 | |||
| 10 ng 重组PLK1和不同浓度Rigosertib在15 µL反应混合物(50 mM HEPES,10 mM MgCl2,1 mM EDTA,2 mM二硫苏糖醇,0.01% NP-40,pH 为7.5)中在室温下反应30分钟。激酶反应在20 µL反应混合物[15 µL 酶 + 抑制剂, 2 µL 1 mM ATP, 2 µL γ32P ATP (40 μci), 和1 µL 重组Cdc25C (100 ng)或酪蛋白 (1 μg) 底物]在30oC下进行20分钟。在20 µL 2× Laemmli buffer中沸腾2分钟终止反应。通过18% SDS-PAGE分离磷酸化的底物。烘干凝胶柱,用X-射线处理3-10分钟。 | ||||
| 细胞实验 | 细胞系 | 多种肿瘤细胞系,包括 BT20,MCF-7,DU145,PC3,U87,A549,H187,RF1,HCT15,HeLa,和Raji细胞 | ||
| 浓度 | 1 nM-10 µM, 溶于DMSO,作为储存液 | |||
| 孵育时间 | 96小时 | |||
| 方法 | 细胞生长在含10%FBS和1 unit/mL青霉素-链霉亲和素溶液的DMEM或RPMI培养基中。肿瘤细胞按每孔1×105个细胞接种在6孔板上,24小时后,加入不同浓度Rigosertib。处理96小时后,测定每孔细胞数。通过台酚蓝染色测定全部存活细胞数。 | |||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | pAbl / Abl / PCrk-L / Crk-L / Cleaved caspase3 / Cleaved PARP / pHistone H2A.X |
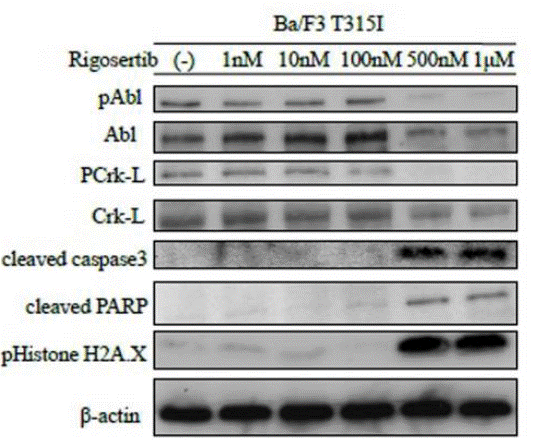
|
26008977 | |
| Immunofluorescence | p-ATF / COX IV |
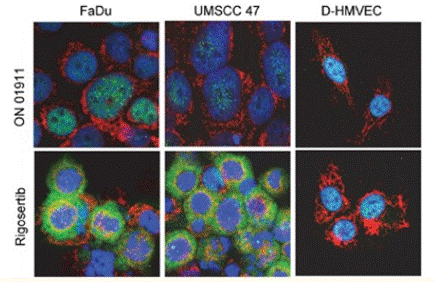
|
27764820 | |
| Growth inhibition assay | GI50 Cell viability |
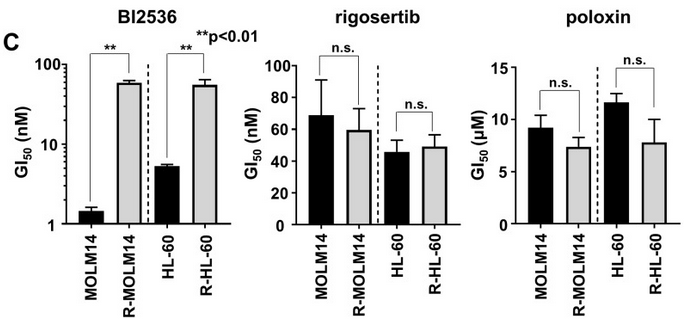
|
29108241 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | Rigosertib按250 mg/kg剂量作用于携带Bel-7402,MCF-7,和MIA-PaCa细胞的鼠移植瘤模型,明显抑制肿瘤生长。Rigosertib按200 mg/kg剂量作用于携带BT20细胞的鼠移植瘤模型,也抑制肿瘤生长。 | |
|---|---|---|
| 动物实验 | Animal Models | 携带Bel-7402,MCF-7,和MIA-PaCa细胞的雌性无胸腺NCR-nu/nu鼠 |
| Dosages | 250 mg/kg | |
| Administration | 腹腔注射 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04177498 | Recruiting | Recessive Dystrophic Epidermolysis Bullosa |
Thomas Jefferson University|Traws Pharma Inc. |
August 24 2021 | Early Phase 1 |
| NCT02075034 | Withdrawn | Myelodysplastic Syndrome |
Traws Pharma Inc. |
May 2014 | Phase 1 |
| NCT02030639 | Completed | Healthy |
Traws Pharma Inc. |
January 2014 | Phase 1 |
| NCT01928537 | Completed | Myelodysplastic Syndromes|Refractory Anemia With Excess Blasts|Chronic Myelomonocytic Leukemia|Cytopenia |
Traws Pharma Inc. |
August 2013 | Phase 3 |
| NCT01807546 | Completed | Head and Neck Squamous Cell Carcinoma|Anal Squamous Cell Carcinoma|Lung Squamous Cell Carcinoma|Cervical Squamous Cell Carcinoma|Esophageal Squamous Cell Carcinoma|Skin Squamous Cell Carcinoma|Penile Squamous Cell Carcinoma |
Traws Pharma Inc. |
March 2013 | Phase 2 |
| NCT01168011 | Completed | Solid Tumor |
Traws Pharma Inc. |
July 2010 | Phase 1 |
|
化学信息&溶解度
| 分子量 | 473.47 | 分子式 | C21H24NNaO8S |
| CAS号 | 1225497-78-8 | SDF | Download Rigosertib (ON-01910) SDF |
| Smiles | COC1=C(C=C(C=C1)CS(=O)(=O)C=CC2=C(C=C(C=C2OC)OC)OC)NCC(=O)[O-].[Na+] | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 95 mg/mL ( (200.64 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : 95 mg/mL (200.64 mM) Ethanol : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项




