- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Sonidegib
别名: NVP-LDE225, Erismodegib 中文名称:索尼德吉
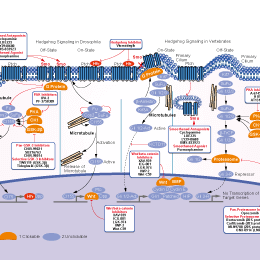
Sonidegib是一种Smoothened(Smo)拮抗剂,抑制Hedgehog (Hh)信号通路,无细胞试验中IC50分别为1.3 nM (小鼠)和2.5 nM(人)。Phase 3。
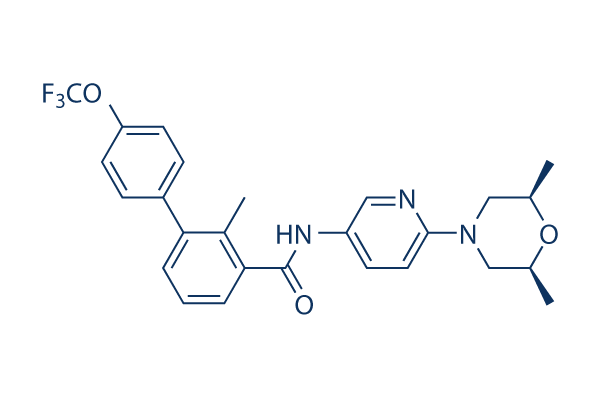
Sonidegib Chemical Structure
CAS: 956697-53-3


产品质控
批次:
纯度:
99.90%
99.90
Sonidegib相关产品
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| UACC 257 | Function assay | 10 μM | inhibits Hedgehog-GLI pathway | 23935925 | |
| LOX IMVI | Function assay | 10 μM | inhibits Hedgehog-GLI pathway | 23935925 | |
| Glioblastoma initiating cells | Function assay | ~10 μM | Inhibits Motility, Invasion, and Migration | 23482671 | |
| Glioblastoma initiating cells | Function assay | ~10 μM | Inhibits the Expression of Genes Involved in Maintaining Pluripotency | 23482671 | |
| Glioblastoma initiating cells | Function assay | ~10 μM | downregulates the SHH signaling pathway | 23482671 | |
| Glioblastoma initiating cells | Cytoxicity assay | ~10 μM | induces apoptosis | 23482671 | |
| Glioblastoma initiating cells | Function assay | ~10 μM | inhibits neurosphere formation | 23482671 | |
| Glioblastoma initiating cells | Cytoxicity assay | ~10 μM | Inhibits Cell Viability | 23482671 | |
| OS18 | Growth inhibitory assay | ~5 μM | reduces the proliferation | 23243595 | |
| OS5 | Growth inhibitory assay | ~5 μM | reduces the proliferation | 23243595 | |
| HeyA8MDR | Cytoxicity assay | ~10 μM | IC50=8 μM | 22553355 | |
| HeyA8 | Cytoxicity assay | ~10 μM | IC50=18 μM | 22553355 | |
| SKOV3TRip2 | Cytoxicity assay | ~10 μM | IC50=12 μM | 22553355 | |
| SKOV3ip1 | Cytoxicity assay | ~10 μM | IC50=24 μM | 22553355 | |
| A2780cp20 | Cytoxicity assay | ~10 μM | IC50=7.5 μM | 22553355 | |
| A2780ip2 | Cytoxicity assay | ~10 μM | IC50=12 μM | 22553355 | |
| LOX IMVI | Function assay | 10 μM | induces G1 cell cycle arrest | 23935925 | |
| UACC 257 | Function assay | 10 μM | induces G1 cell cycle arrest | 23935925 | |
| LOX IMVI | Cytoxicity assay | 10 μM | decreases tumor cell viability | 23935925 | |
| UACC 257 | Cytoxicity assay | 10 μM | decreases tumor cell viability | 23935925 | |
| LOX IMVI | Apoptosis assay | 10 μM | induces apoptosis | 23935925 | |
| UACC 257 | Apoptosis assay | 10 μM | induces apoptosis | 23935925 | |
| ACHN | Growth inhibitory assay | ~5 μM | IC50=2-3 μM | 25093491 | |
| 769-P | Growth inhibitory assay | ~5 μM | IC50=2-3 μM | 25093491 | |
| 786-O | Growth inhibitory assay | ~5 μM | IC50=2-3 μM | 25093491 | |
| 786-O SuR | Growth inhibitory assay | ~5 μM | IC50=2-3 μM | 25093491 | |
| SP53 | Function assay | 30 μM | inhibits cell adhesion and migration | 26885608 | |
| SP53 | Function assay | 30 μM | inhibits the VLA4-mediated FAK signaling pathway | 26885608 | |
| HS5 | Function assay | 30 μM | inhibits cell adhesion and migration | 26885608 | |
| HS27a | Function assay | 30 μM | inhibits cell adhesion and migration | 26885608 | |
| SP53 | Cytoxicity assay | 30 μM | induces autophagy | 26885608 | |
| Jeko | Cytoxicity assay | 30 μM | induces autophagy | 26885608 | |
| U2OS | Function assay | 2 hrs | Displacement of [3H]cyclopamine from wild type Smo expressed in U2OS cells after 2 hrs by scintillation counting, Ki = 0.006 μM. | 23063522 | |
| NIH/3T3 | Function assay | 24 hrs | Inhibition of hedgehog signaling pathway in mouse NIH/3T3 cells measured after 24 hrs by Gli-dual luciferase reporter gene assay, IC50 = 0.006 μM. | 27810591 | |
| TM3 | Function assay | 48 hrs | Inhibition of Hh signaling pathway in mouse TM3 cells assessed as downregulation of Gli1 gene expression after 48 hrs by luciferase reporter gene assay, EC50 = 0.0012 μM. | 26976215 | |
| NIH3T3 | Function assay | 48 hrs | Inhibition of SHH signaling pathway in mouse NIH3T3 cells measured after 48 hrs by Gli-luciferase reporter assay, IC50 = 0.0055 μM. | 24176396 | |
| NIH3T3 | Function assay | 48 hrs | Inhibition of Sonic-induced hedgehog signalling in mouse NIH3T3 cells after 48 hrs by Gli-luciferase reporter assay, IC50 = 0.0055 μM. | 26820554 | |
| NIH/3T3 | Function assay | Inhibition of hedgehog signaling pathway in mouse NIH/3T3 cells expressing wild type Smo assessed as reduction in Gli mRNA expression by RT-PCR method, IC50 = 0.0044 μM. | 27810591 | ||
| NIH/3T3 | Function assay | Inhibition of hedgehog signaling pathway in mouse NIH/3T3 cells harboring Smo D477H mutant assessed as reduction in Gli mRNA expression by RT-PCR method, IC50 = 0.0227 μM. | 27810591 | ||
| NIH/3T3 | Function assay | Inhibition of hedgehog signaling pathway expressed in mouse NIH/3T3 cells assessed as inhibition of hedgehog-induced Gli-2 accumulation at tip of primary cilia by DAPI staining based confocal microscopic analysis | 27810591 | ||
| NIH/3T3 | Function assay | Inhibition of hedgehog signaling pathway in mouse NIH/3T3 cells assessed as inhibition of hedgehog-induced Smo-EGFP ciliary translocation by DAPI staining based confocal microscopic analysis | 27810591 | ||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | ||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | ||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | ||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | ||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | ||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | ||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | ||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | ||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | ||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | ||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | ||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Sonidegib是一种Smoothened(Smo)拮抗剂,抑制Hedgehog (Hh)信号通路,无细胞试验中IC50分别为1.3 nM (小鼠)和2.5 nM(人)。Phase 3。 | ||||
|---|---|---|---|---|---|
| 特性 | LDE225 是具有高效性和选择性的使平滑的拮抗剂 | ||||
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | Sonidegib (Erismodegib, NVP-LDE225)可在0.6-0.8μM剂量上抑制1nM-25nM Hh激动剂Ag1.5 处理的TM3荧光报告细胞系。[1] | |||
|---|---|---|---|---|
| 细胞实验 | 细胞系 | TM3Hh12 细胞 | ||
| 浓度 | 0 μM -10 μM | |||
| 孵育时间 | 30 分钟 | |||
| 方法 | LDE225 用DMSO连续稀释后加入空的分析平板中。TM3Hh12 细胞 (TM3 细胞含有Hh的应答报告基因元件pTA-8xGli-Luc) 用含有5%的马血清,2.5%的胎牛血清(FBS)和15 mM HEPES, pH 7.3的F12 Ham’s/DMEM (1:1)培养基培养。收集细胞时用胰酶消化,然后用含有5%的马血清和15 mM HEPES, pH 7.3的F12 Ham’s/DMEM (1:1)培养基重悬,接着分铺到分析板中。然后加入LDE225在37 °C ,5% CO2的培养箱中大约孵育30分钟。接着加入1 nM 和25 nM 的Ag1.5到分析平板中,37 °C ,5% CO2的条件下培养。48小时后,向平板中加入Bright-Glo 或者 MTS试剂,测量492纳米下的荧光值或者吸收峰。通过MTS法检测Gli-驱动荧光素酶发光或吸光度信号,对浓度取Log(10)值做由非线性回归曲线,确定IC 50值。数据处理使用R统计软件包。 |
|||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | p-p130CAS / p130CAS / p-FAK / FAK / p-Paxillin / Paxillin pp70S6K / P70s6k Gli-1 / Gli-2 / p-Akt / Akt Bcl2 / c-myc / Cyclin D1 / pERK / ERK / pJNK / JNK / Caspase 3 / Cleaved caspase 3 |
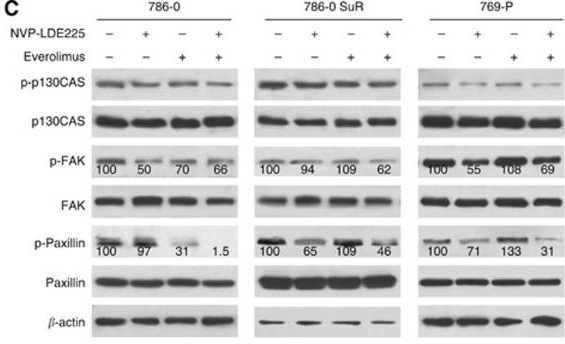
|
25093491 | |
| Immunofluorescence | GLI1 |
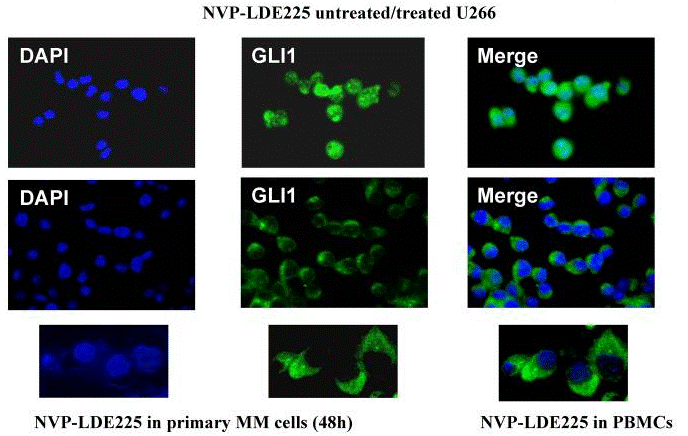
|
22821765 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | Sonidegib (Erismodegib, NVP-LDE225)与小鼠,大鼠以及人源的血浆蛋白有很强的结合能力(>99%),同时与狗和猴子的血浆蛋白有适度的结合,结合能力分别是77 %和 85%。PAMPA 实验证明LDE225能够达到90.8%的渗透性。在梯度稀释的试验中,LDE225在临床物种上显示出很好的口服药效率,其生物药效率在69%到102%之间。LDE225呈弱碱性,pKa只有4.20,同时它的水溶性也相对较弱。LDE225被证明具有剂量依赖的抗肿瘤活性。给药剂量在5 mg/kg/天,一天一次时,LDE225明显抑制肿瘤生长,与33%的T/C值相一致。给药剂量在10 mg/kg/天,一天一次和 20 mg/kg/天,一天一次时,LDE225促使肿瘤退化的效果分别达到51%和83%。Gli1 mRNA抑制性与LDE225介导的肿瘤与血浆接触有关。在肿瘤移植的动物模型中,经过四天的给药处理,LDE225能够成功地穿过血脑屏障导致肿瘤生长受抑制[1]。LDE225能够使Rip1-Tag2小鼠中的肿瘤体积明显减少95.7%。LDE225减少LDE225给药处理小鼠的间质状标志基因的表达[2]。 | |
|---|---|---|
| 动物实验 | Animal Models | 采用无胸腺裸小鼠建立原位Ptch+/-p53-/-髓母细胞瘤同种异体移植模型 |
| Dosages | 40 mg/kg/天 | |
| Administration | 口服,每天两次 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02358161 | Completed | Pancreatic Cancer |
Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Novartis|Celgene Corporation |
September 2015 | Phase 1|Phase 2 |
| NCT02254551 | Terminated | Multiple Myeloma |
SCRI Development Innovations LLC|Novartis |
January 2015 | Phase 2 |
| NCT02138929 | Completed | Esophageal Cancer |
M.D. Anderson Cancer Center|Novartis|National Cancer Institute (NCI) |
November 10 2014 | Phase 1 |
| NCT02195973 | Completed | Recurrent Ovarian Cancer |
University of Alabama at Birmingham|Novartis Pharmaceuticals |
September 2014 | Phase 1 |
| NCT02027376 | Completed | Advanced Breast Cancer |
Spanish Breast Cancer Research Group|Novartis |
May 2014 | Phase 1 |
| NCT02111187 | Completed | Prostate Cancer |
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins |
April 2014 | Phase 1 |
|
化学信息&溶解度
| 分子量 | 485.5 | 分子式 | C26H26F3N3O3 |
| CAS号 | 956697-53-3 | SDF | Download Sonidegib SDF |
| Smiles | CC1CN(CC(O1)C)C2=NC=C(C=C2)NC(=O)C3=CC=CC(=C3C)C4=CC=C(C=C4)OC(F)(F)F | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 97 mg/mL ( (199.79 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 16 mg/mL (32.95 mM) Water : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项