- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Fexagratinib (AZD4547)
别名: ABSK 091
Fexagratinib (AZD4547,ABSK 091)是一种新型选择性的FGFR抑制剂,靶向作用于FGFR1/2/3,在无细胞试验中IC50为0.2 nM/2.5 nM/1.8 nM,对FGFR4, VEGFR2(KDR)具有微弱的作用活性,对IGFR, CDK2和p38几乎没有作用活性。Phase 2/3。
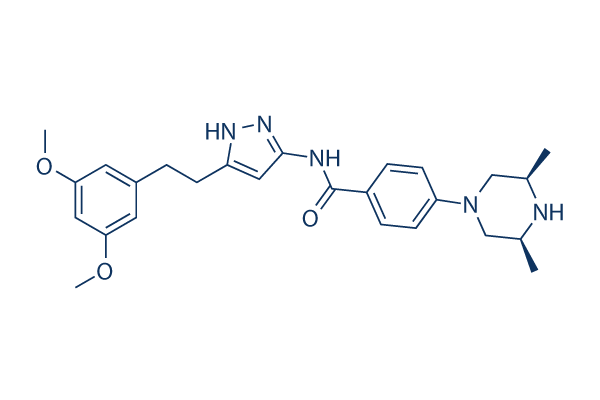
Fexagratinib (AZD4547) Chemical Structure
CAS: 1035270-39-3


产品质控
批次:
纯度:
99.94%
99.94
Fexagratinib (AZD4547) 相关产品
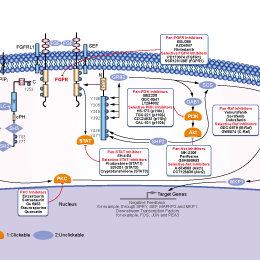
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| SK-HEP-1 | Function Assay | 0-2 μM | 48 h | causes a decrease of FRS2,AKT, and ERK phosphorylation | 26351320 |
| SNU449 | Function Assay | 0-2 μM | 48 h | causes a decrease of FRS2,AKT, and ERK phosphorylation | 26351320 |
| SK-HEP-1 | Clonogenic assay | 1 µM | 24 h | decreases colony formation significantly | 26351320 |
| SNU449 | Clonogenic assay | 1 µM | 24 h | decreases colony formation significantly | 26351320 |
| A549 | Cell viability Assay | 0.1/1 μM | 48 h | enhances Erlotinib induced viability loss | 26053020 |
| SGC-7901 | Growth Inhibition Assay | 1 nM-10 μM | 72 h | IC50 of 5-10 μM, inhibits cell viability dose dependently | 25576915 |
| HGC-27 | Growth Inhibition Assay | 1 nM-10 μM | 72 h | IC50 of 5-10 μM, inhibits cell viability dose dependently | 25576915 |
| MKN-28 | Growth Inhibition Assay | 1 nM-10 μM | 72 h | IC50 of 5-10 μM, inhibits cell viability dose dependently | 25576915 |
| NCI-N87 | Growth Inhibition Assay | 1 nM-10 μM | 72 h | IC50 of 5-10 μM, inhibits cell viability dose dependently | 25576915 |
| KATOIII | Growth Inhibition Assay | 1 nM-10 μM | 72 h | IC50 of 10-100 nM, inhibits cell viability dose dependently | 25576915 |
| SNU-16 | Growth Inhibition Assay | 1 nM-10 μM | 72 h | IC50 of 10-100 nM, inhibits cell viability dose dependently | 25576915 |
| 4T1 | Apoptosis Assay | 1.25-20 μM | 24 h | induces apoptosis dose dependently | 24642893 |
| HepG2 | Growth Inhibition Assay | 72 h | IC50=8.73 μM | 26351320 | |
| Hur7 | Growth Inhibition Assay | 72 h | IC50=7.25 μM | 26351320 | |
| PLC/PRF5 | Growth Inhibition Assay | 72 h | IC50=6.55 μM | 26351320 | |
| Hep3B | Growth Inhibition Assay | 72 h | IC50=6.43 μM | 26351320 | |
| SNU475 | Growth Inhibition Assay | 72 h | IC50=5.4 μM | 26351320 | |
| SK-HEP-1 | Growth Inhibition Assay | 72 h | IC50=0.084 μM | 26351320 | |
| SNU449 | Growth Inhibition Assay | 72 h | IC50=0.082 μM | 26351320 | |
| KG1 | Function assay | 72 hrs | Inhibition of FGFR1 in human KG1 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0002 μM. | 27117427 | |
| RT112 | Function assay | 72 hrs | Inhibition of FGFR3 in human RT112 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0006 μM. | 27117427 | |
| KG1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KG1 cells expressing FGFR1 after 72 hrs, IC50 = 0.0019 μM. | 29775937 | |
| KG1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KG1 cells after 72 hrs by CCK-8 assay, IC50 = 0.0033 μM. | 28687204 | |
| SNU16 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SNU16 cells after 72 hrs by CCK-8 assay, IC50 = 0.0034 μM. | 28687204 | |
| SNU16 | Function assay | 72 hrs | Inhibition of recombinant FGFR2 in human SNU16 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0036 μM. | 27117427 | |
| SNU16 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SNU16 cells expressing FGFR2 after 72 hrs, IC50 = 0.0062 μM. | 29775937 | |
| KG1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against FGFR1-translocated human KG1 cells after 72 hrs by CCK8/MTT assay, IC50 = 0.0064 μM. | 27348537 | |
| SNU16 | Antiproliferative assay | 72 hrs | Antiproliferative activity against FGFR2-amplified human SNU16 cells after 72 hrs by CCK8/MTT assay, IC50 = 0.0134 μM. | 27348537 | |
| KATO III | Function assay | 72 hrs | Inhibition of FGFR2 in human KATO III cells assessed as inhibition of cell proliferation after 72 hrs by cell counting kit-8 assay, IC50 = 0.0141 μM. | 28714692 | |
| KG1 | Function assay | 72 hrs | Inhibition of FGFR1OP2 fused FGFR1 in human KG1 cells assessed as inhibition of cell proliferation after 72 hrs by cell counting kit-8 assay, IC50 = 0.0161 μM. | 28714692 | |
| KATO III | Function assay | 72 hrs | Inhibition of FGFR2 in human KATO III cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0202 μM. | 27117427 | |
| SNU16 | Function assay | 72 hrs | Inhibition of FGFR2 in human SNU16 cells assessed as inhibition of cell proliferation after 72 hrs by cell counting kit-8 assay, IC50 = 0.0254 μM. | 28714692 | |
| UM-UC-14 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human UM-UC-14 cells expressing FGFR3 after 72 hrs, IC50 = 0.0269 μM. | 29775937 | |
| RT112 | Function assay | 72 hrs | Inhibition of FGFR3 in human RT112 cells assessed as inhibition of cell proliferation after 72 hrs by cell counting kit-8 assay, IC50 = 0.027 μM. | 28714692 | |
| UM-UC-14 | Function assay | 72 hrs | Inhibition of FGFR3 mutant in human UM-UC-14 cells assessed as inhibition of cell proliferation after 72 hrs by cell counting kit-8 assay, IC50 = 0.0271 μM. | 28714692 | |
| RT112 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human RT112 cells after 72 hrs by MTT assay, GI50 = 0.0292 μM. | 27599742 | |
| H1581 | Function assay | 72 hrs | Inhibition of FGFR1 in human H1581 cells assessed as suppression of cell proliferation after 72 hrs by SRB/CCK-8 assay, IC50 = 0.0329 μM. | 27117427 | |
| NCI-H1581 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1581 cells expressing FGFR1 after 72 hrs, IC50 = 0.04 μM. | 29775937 | |
| B16F10 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse B16F10 cells after 72 hrs by MTT assay, IC50 = 0.051 μM. | 25736993 | |
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 0.062 μM. | 25736993 | |
| NCI-H1581 | Function assay | 72 hrs | Inhibition of FGFR1 in human NCI-H1581 cells assessed as inhibition of cell proliferation after 72 hrs by cell counting kit-8 assay, IC50 = 0.0626 μM. | 28714692 | |
| RT112 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human RT112 cells expressing FGFR3 after 72 hrs, IC50 = 0.0838 μM. | 29775937 | |
| RT112 | Antiproliferative assay | 72 hrs | Antiproliferative activity against FGFR3-amplified human RT112 cells after 72 hrs by CCK8/MTT assay, IC50 = 0.0884 μM. | 27348537 | |
| NCI-H1581 | Antiproliferative assay | 72 hrs | Antiproliferative activity against FGFR1-amplified human NCI-H1581 cells after 72 hrs by CCK8/MTT assay, IC50 = 0.0921 μM. | 27348537 | |
| HeLa 229 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa 229 cells after 72 hrs by MTT assay, IC50 = 0.14 μM. | 25736993 | |
| BAF3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BAF3 cells expressing VEGFR2 after 72 hrs, IC50 = 0.222 μM. | 29775937 | |
| SGC7901 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SGC7901 cells after 72 hrs by MTT assay, IC50 = 2.8 μM. | 27829519 | |
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50 = 6.2 μM. | 27829519 | |
| BGC823 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human BGC823 cells after 72 hrs by MTT assay, IC50 = 7.9 μM. | 27829519 | |
| HL7702 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HL7702 cells after 72 hrs by MTT assay, IC50 = 18.21 μM. | 25736993 | |
| HEC1A Normal FGFR2 | Growth Inhibition Assay | GI50﹥10 μM | 26294741 | ||
| Ishikawa FGFF2 over exp. | Growth Inhibition Assay | GI50=4.5 ± 1.51 μM | 26294741 | ||
| MFE280 FGFR2 S252W | Growth Inhibition Assay | GI50=0.218 ± 0.073 μM | 26294741 | ||
| MFE296 FGFR2 N550K | Growth Inhibition Assay | GI50=0.730 ± 0.057 μM | 26294741 | ||
| AN3-CA FGFR2 N550K, K310R | Growth Inhibition Assay | GI50=0.031 ± 0.023 μM | 26294741 | ||
| TT RET C634W | Growth Inhibition Assay | GI50=2.9 ± 0.904 μM | 26294741 | ||
| MV4-11 FLT3/ITD | Growth Inhibition Assay | GI50=0.459 ± 0.046 μM | 26294741 | ||
| MOLM14 FLT3/ITD | Growth Inhibition Assay | GI50=0.484 ± 0.157 μM | 26294741 | ||
| BaF3 Parental | Growth Inhibition Assay | GI50﹥10 μM | 26294741 | ||
| BaF3 RET-TEL | Growth Inhibition Assay | GI50=0.39 ± 0.048 μM | 26294741 | ||
| BaF3 FLT3-TEL | Growth Inhibition Assay | GI50=4.6 ± 0.577 μM | 26294741 | ||
| 4T1 | Growth Inhibition Assay | IC50=0.64±0.11 μM | 24642893 | ||
| MDA-MB-468 | Growth Inhibition Assay | IC50=4.9±0.85 μM | 24642893 | ||
| HCT116 | Growth Inhibition Assay | IC50=15.9±1.82 μM | 24642893 | ||
| SW620 | Growth Inhibition Assay | IC50>20 μM | 24642893 | ||
| MDA-MB-231 | Growth Inhibition Assay | IC50>20 μM | 24642893 | ||
| CT26 | Growth Inhibition Assay | IC50>20 μM | 24642893 | ||
| SW480 | Growth Inhibition Assay | IC50>20 μM | 24642893 | ||
| KG1a | Growth Inhibition Assay | IC50=0.018 μM | 22369928 | ||
| Sum52-PE | Growth Inhibition Assay | IC50=0.041 μM | 22369928 | ||
| KMS11 | Growth Inhibition Assay | IC50=0.281 μM | 22369928 | ||
| MCF7 | Growth Inhibition Assay | IC50>30 μM | 22369928 | ||
| U-2 OS | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | ||
| A673 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | ||
| Saos-2 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | ||
| BT-37 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | ||
| RD | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | ||
| SK-N-SH | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | ||
| BT-12 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | ||
| OHS-50 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | ||
| fibroblast cells | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for control Hh wild type fibroblast cells | 29435139 | ||
| Rh41 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | ||
| Rh30 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | ||
| SJ-GBM2 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | ||
| NB-EBc1 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | ||
| LAN-5 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | ||
| Rh18 | qHTS ssay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Fexagratinib (AZD4547,ABSK 091)是一种新型选择性的FGFR抑制剂,靶向作用于FGFR1/2/3,在无细胞试验中IC50为0.2 nM/2.5 nM/1.8 nM,对FGFR4, VEGFR2(KDR)具有微弱的作用活性,对IGFR, CDK2和p38几乎没有作用活性。Phase 2/3。 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 特性 | AZD4547高选择性作用于 FGFR1-3,比作用于FGFR4选择性高。AZD4547有效作用于野生型和突变型FGFR酪氨酸激酶活性。 | ||||||||
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | 与 FGFR1-3相比, AZD4547作用于FGFR4,活性微弱,IC50为165 nM。AZD4547 只抑制重组 VEGFR2 (KDR) 激酶活性, IC50为 24 nM,在体外选择性作用于一组多种代表性的人类激酶。0.1 μM AZD4547 作用于一系列重组激酶,包括 ALK, CHK1, EGFR, MAPK1, MEK1, p70S6K, PDGFR, PKB, Src, Tie2,和 PI3K,没有作用活性。相应地,在细胞磷酸化实验中,可观察到AZD4547 作用于FGFR1-3的选择性比作用于 FGFR4, IGFR, 和 KDR高。AZD4547在体外,只有作用于表达去调控FGFRs 如KG1a, Sum52-PE,和KMS11的肿瘤细胞,具有有效抗增殖活性,IC50 为18-281 nM,而对MCF7 及100 种以上其他肿瘤细胞无活性。AZD4547 处理人类肿瘤细胞,有效抑制FGFR 和 MAPK 磷酸化,这种作用存在剂量依赖性。AZD4547 也有效抑制FRS2 和 PLCγ磷酸化,及下游FGFR信号。另外, AZD4547作用于乳腺细胞系,MCF7和Sum52-PE 而不是KG1a和 KMS11细胞,影响AKT磷酸化。AZD4547处理Sum52-PE 和 KMS11细胞,显著诱导凋亡,作用于KG1a细胞,显著提高细胞周期在G1期停滞而不是凋亡, 而作用于MCF7细胞,对细胞周期分布和凋亡都没有作用效果。 |
|||
|---|---|---|---|---|
| 激酶实验 | AZD4547 激酶活性 | |||
| 使用浓度等于或低于相对Km 的ATP,测定AZD4547抑制 FGFR1-3的人类重组激酶活性的效果。 | ||||
| 细胞实验 | 细胞系 | KG1a, Sum52-PE, KMS11, 和MCF7 | ||
| 浓度 | 溶于DMSO,终浓度为 ~1 μM | |||
| 孵育时间 | 72 小时 | |||
| 方法 | 使用多种浓度AZD4547处理细胞72小时。通过 MTS 增殖实验获得抗增殖的IC50值。荧光激活细胞分选(FACS)中,细胞与70%乙醇混合,然后与碘化丙啶/RNase A标记溶液温育。使用 FACSCalibur 仪器和CellQuest分析软件测定细胞周期谱。为了分析凋亡,轻轻收集细胞和培养基,离心,然后冲洗细胞颗粒。细胞进行Annexin膜联蛋白 V-异硫氰酸荧光素(FITC)染色和碘化丙啶吸收。使用FACSCalibur仪器测定 膜联蛋白 V染色阳性细胞比例,使用CellQuest分析软件进行象限分类。 |
|||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | p-FGFR / FGFR1 / p-AKT / AKT / p-ERK / ERK pFRS2 |
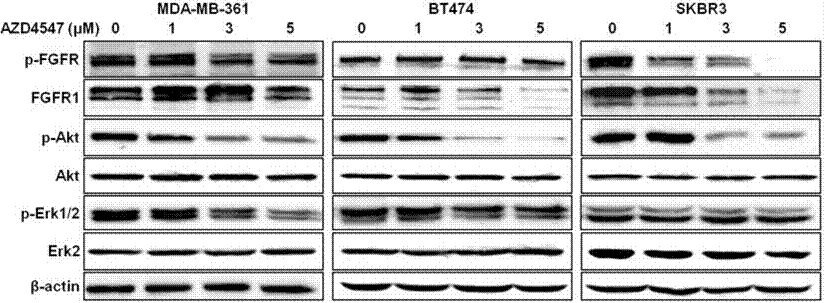
|
28900173 | |
| Growth inhibition assay | Cell viability Cell viability |
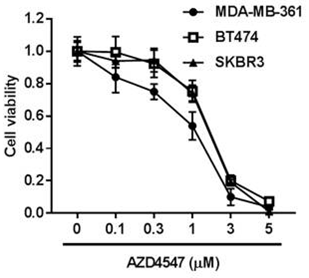
|
28900173 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | AZD4547按3 mg/kg剂量口服处理携带KMS11肿瘤的小鼠,每天两次,与空白对照组相比,显著抑制53%肿瘤生长,AZD4547 按 12.5 mg/kg剂量每天处理一次,或按 6.25 mg/kg剂量每天处理两次,则完全抑制肿瘤,这与p-FGFR3的药效学调节剂量正相关,且降低 KMS11肿瘤细胞增殖。而且, AZD4547按 12.5 mg/kg剂量口服处理给药FGFR1融合KG1a 移植瘤模型,每天一次,抑制65% 肿瘤生长。在有效剂量水平,AZD4547不表现出抗血管生成的效果。AZD4547 对血压没有显著作用效果,因此在体内缺乏 抗-KDR 活性。相应地, AZD4547按6.25 mg/kg剂量口服处理对Cediranib敏感的移植瘤模型,包括 Calu-6, HCT-15 和 LoVo,每天两次,没有作用活性。 |
|
|---|---|---|
| 动物实验 | Animal Models | 皮下注射LoVo, HCT-15, Calu-6, KMS11 或 KG1a的雌性Swiss衍生裸鼠和SCID小鼠 |
| Dosages | 1.5-50 mg/kg | |
| Administration | 口服饲喂,每天一次或两次 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02824133 | Completed | Recurrent IDHwt Gliomas With FGFR3-TACC3 Fusion|Recurrent IDHwt Gliomas With FGFR1-TACC1 Fusion |
Assistance Publique - Hôpitaux de Paris |
September 2015 | Phase 1|Phase 2 |
| NCT01824901 | Completed | Recurrent Non-small Cell Lung Cancer|Squamous Cell Lung Cancer |
ECOG-ACRIN Cancer Research Group|National Cancer Institute (NCI)|Eastern Cooperative Oncology Group |
January 15 2014 | Phase 1|Phase 2 |
| NCT01795768 | Unknown status | Gastric Cancer|Oesophageal Cancer|Breast Cancer|Squamous Cell Carcinoma of the Lung |
Royal Marsden NHS Foundation Trust|AstraZeneca |
September 2012 | Phase 2 |
| NCT01213160 | Completed | Cancer|Advanced Solid Malignancies |
AstraZeneca |
November 2010 | Phase 1 |
|
化学信息&溶解度
| 分子量 | 463.57 | 分子式 | C26H33N5O3 |
| CAS号 | 1035270-39-3 | SDF | Download Fexagratinib (AZD4547) SDF |
| Smiles | CC1CN(CC(N1)C)C2=CC=C(C=C2)C(=O)NC3=NNC(=C3)CCC4=CC(=CC(=C4)OC)OC | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 93 mg/mL ( (200.61 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项