- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Ruxolitinib (INCB18424)
别名: INCB018424 中文名称:鲁索替尼
Ruxolitinib是第一个应用于临床的,有效的,选择性JAK1/2抑制剂,在无细胞试验中IC50为3.3 nM/2.8 nM。作用于JAK1, JAK2与作用于JAK3相比,选择性高130多倍。Ruxolitinib 通过毒性线粒体自噬杀死肿瘤细胞。Ruxolitinib 可诱导自噬并增强细胞凋亡。
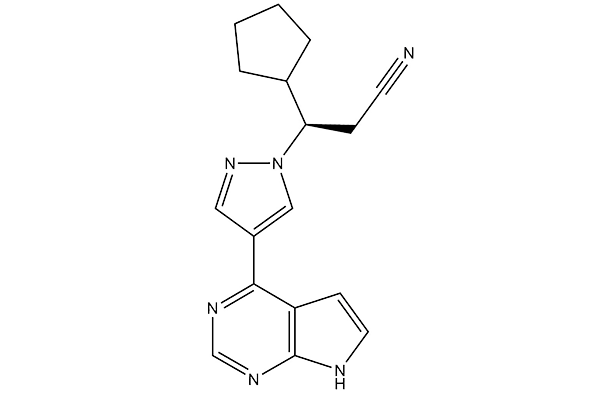
Ruxolitinib (INCB18424) Chemical Structure
CAS: 941678-49-5


产品质控
批次:
纯度:
99.98%
99.98
常与Ruxolitinib (INCB18424)一起在实验中被使用的化合物
Ruxolitinib (INCB18424)相关产品
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| A549/DDP | Function Assay | 30 nM | 48 h | Down-regulation of STAT3 phosphorylation | 25213670 |
| NCI-H2347 | Function Assay | 30 nM | 48 h | Decrease in Bcl2 expression | 25213670 |
| NCI-H1299 | Function Assay | 30 nM | 48 h | Down-regulation of STAT3 phosphorylation | 25213670 |
| A549/DDP | Apoptosis Assay | 30 nM | 48 h | Induction of apoptosis | 25213670 |
| NCI-H1299 | Apoptosis Assay | 30 nM | 48 h | Induction of apoptosis | 25213670 |
| NCI-H2347 | Apoptosis Assay | 30 nM | 48 h | Induction of apoptosis | 25213670 |
| Hep3B | Function Assay | 1 μM | 16 h | Impaires the capacity of IHCA-associated gp130 mutants to active STAT3 with IC50 of ~50 μM | 24501689 |
| HepG2 | Function Assay | 1 μM | 16 h | Impaires the capacity of IHCA-associated gp130 mutants to signal to STAT3 | 24501689 |
| Huh7 | Function Assay | 1 μM | 16 h | Impaires the capacity of IHCA-associated gp130 mutants to signal to STAT3 | 24501689 |
| BaF3 | Kinase Assay | 80 nM | 6 h | Reduces the phosphorylation of STAT5 in JAK2V617F-mutated BAF3-EPOR cell | 24237791 |
| DLD-1 | Kinase Assay | 25 μM | 48 h | Inhibition of JAK1 phosphorylation | 24050550 |
| RKO | Kinase Assay | 25 μM | 48 h | Inhibition of JAK1 phosphorylation | 24050550 |
| DLD-1 | Kinase Assay | 25 μM | 48 h | Inhibition of JAK2 phosphorylation | 24050550 |
| RKO | Kinase Assay | 25 μM | 48 h | does not inhibit JAK1 phosphorylation | 24050550 |
| RKO | Growth Inhibition Assay | 50 μM | 48 h | IC50=14.76 μM | 24050550 |
| DLD-1 | Growth Inhibition Assay | 50 μM | 48 h | IC50=15.51 μM | 24050550 |
| DLD-1 | Apoptosis Assay | 25 μM | 48 h | Induces apoptosis by activating caspase 3 | 24050550 |
| RKO | Apoptosis Assay | 25 μM | 48 h | Induces apoptosis by activating caspase 3 | 24050550 |
| HuH7 | Growth Inhibition Assay | 50 μM | 48 h | >82% reduction | 23941832 |
| SNU182 | Growth Inhibition Assay | 50 μM | 48 h | >64% reduction | 23941832 |
| SNU423 | Growth Inhibition Assay | 50 μM | 48 h | >81% reduction | 23941832 |
| HuH7 | Function Assay | 50 μM | 24 h | Inhibition of STAT1 and STAT3 phosphorylation significantly | 23941832 |
| SNU182 | Function Assay | 50 μM | 24 h | Inhibition of STAT1 and STAT3 phosphorylation significantly | 23941832 |
| SNU423 | Function Assay | 50 μM | 24 h | Inhibition of STAT1 and STAT3 phosphorylation significantly | 23941832 |
| HT93A | Growth Inhibition Assay | 320 nM | 5 d | Inhibition of GCS-F induced granulocytic differentiation | 25805962 |
| SET-2 | Cytotoxic Assay | 5 μM | 48 h | Cytotoxic index=18.7% | 25931349 |
| HEL | Cytotoxic Assay | 5 μM | 48 h | Cytotoxic index=12.2% | 25931349 |
| TF1 | Kinase Assay | 20 min | Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation with IC50 of 0.024μM | 22698084 | |
| TF1 | Kinase Assay | 20 min | Inhibition of JAK2 in human TF1 cells assessed as inhibition of EPO-induced STAT5 phosphorylation with IC50 of 0.012μM | 22698084 | |
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0001 μM | 23668484 | |
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0002 μM | 23668484 | |
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0005 μM | 23668484 | |
| Sf21 cells | JAK inhibition assay | 1 h | IC50 = 0.0028 μM | 22591402 | |
| Sf21 cells | JAK inhibition assay | 60 min | IC50 = 0.003 μM | 27137359 | |
| Sf9 cells | JAK inhibition assay | 1 h | Ki = 0.0032 μM | 23668484 | |
| Sf21 cells | JAK inhibition assay | 1 h | IC50 = 0.0033 μM | 22591402 | |
| TF1 cells | JAK inhibition assay | 30 min | IC50 = 0.00685 μM | 23061660 | |
| TF1 cells | JAK inhibition assay | 20 min | EC50 = 0.012 μM | 22698084 | |
| Sf21 cells | TYK2 inhibition assay | 1 h | IC50 = 0.019 μM | 22591402 | |
| TF1 cells | JAK inhibition assay | 20 min | EC50 = 0.024 μM | 22698084 | |
| Sf21 cells | JAK inhibition assay | 1 h | IC50 = 0.428 μM | 22591402 | |
| CD34+ cells | JAK inhibition assay | 45 min | IC50 = 0.677 μM | 24417533 | |
| NCI-H2347 | Growth Inhibition Assay | IC50=0.17 μM | 25213670 | ||
| NCI-H1299 | Growth Inhibition Assay | IC50=0.28 μM | 25213670 | ||
| A549/DDP | Growth Inhibition Assay | IC50=0.22 μM | 25213670 | ||
| A549 | Growth Inhibition Assay | IC50=0.04 μM | 25213670 | ||
| NCI-H358 | Growth Inhibition Assay | IC50=0.1 μM | 25213670 | ||
| NCI-H460 | Growth Inhibition Assay | IC50=0.13 μM | 25213670 | ||
| CMK | Growth Inhibition Assay | Inhibition of CMK carrying the WT JAK cell proliferation with IC50 of 0.075 μM | 25352124 | ||
| CMK | Growth Inhibition Assay | Inhibition of CMK carrying the JAK3A63D mutation cell proliferation with IC50 of 0.163 μM | 25352124 | ||
| CMK | Growth Inhibition Assay | Inhibition of CMK carrying the JAK3A572V mutation cell proliferation | 25352124 | ||
| Human monocyte | Kinase Assay | Inhibition of JAK2/1 in human monocytes expressing CD14 assessed as inhibition of IFNgamma-stimulated STAT1 phosphorylation with IC50 of 0.031μM | 23540648 | ||
| Human monocyte | Kinase Assay | Inhibition of JAK2 in human monocytes expressing CD14 assessed as inhibition of GM-CSF-stimulated STAT5a phosphorylation with IC50 of 0.026μM | 23540648 | ||
| Human T cell | Kinase Assay | Inhibition of JAK3/1 in human T cells expressing CD3 assessed as inhibition of IL2-stimulated STAT5a phosphorylation with IC50 of 0.023μM | 23540648 | ||
| SET2 cells | JAK inhibition assay | IC50 = 0.00184 μM | 23061660 | ||
| CD34+ cells | JAK inhibition assay | IC50 = 0.008 μM | 26927423 | ||
| T cells | JAK inhibition assay | IC50 = 0.023 μM | 23540648 | ||
| T cells | JAK inhibition assay | IC50 = 0.023 μM | 23540648 | ||
| T cells | JAK inhibition assay | IC50 = 0.031 μM | 23540648 | ||
| T cells | JAK inhibition assay | IC50 = 0.031 μM | 23540648 | ||
| PBMC cells | JAK inhibition assay | IC50 = 0.04 μM | 26927423 | ||
| PBMC cells | STAT5 inhibition assay | IC50 = 0.448 μM | 26927423 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Ruxolitinib是第一个应用于临床的,有效的,选择性JAK1/2抑制剂,在无细胞试验中IC50为3.3 nM/2.8 nM。作用于JAK1, JAK2与作用于JAK3相比,选择性高130多倍。Ruxolitinib 通过毒性线粒体自噬杀死肿瘤细胞。Ruxolitinib 可诱导自噬并增强细胞凋亡。 | ||||
|---|---|---|---|---|---|
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | 在Ba/F3细胞和HEL细胞中,INCB018424有效地和有选择性地抑制JAK2V617F介导的信号传导和细胞增殖。INCB018424以剂量依赖性的方式显着地增加Ba/F3细胞的细胞凋亡。在Ba/F3细胞中,INCB018424(64 nM)致使线粒体去极化细胞增加一倍。INCB018424抑制来自正常捐助者和真性红细胞增多症患者的红细胞前体细胞的增殖,IC50分别是407 nM 和223 nM。 INCB018424有效抑制红细胞集落形成,IC50是67 nM。[1] | |||
|---|---|---|---|---|
| 激酶实验 | 结合试验 | |||
| 重组蛋白是使用Sf21细胞和杆状病毒载体表达的,并通过亲和层析纯化。JAK激酶测定使用肽底物(-EQEDEPEGDYFEWLE)的均相时间分辨荧光测定法。酶反应是用Ruxolitinib或对照,JAK酶,500 nM肽,三磷酸腺苷(ATP; 1mM),和2%的二甲基亚砜(DMSO)反应1小时。 50%抑制浓度(IC50)时需要抑制50%荧光信号的INCB018424浓度。 | ||||
| 细胞实验 | 细胞系 | Ba/F3和HEL细胞 | ||
| 浓度 | 3 μM | |||
| 孵育时间 | 48小时 | |||
| 方法 | 2×103细胞接种于的96孔板的一个孔中,用溶于DMSO的INCB018424(0.2%DMSO终浓度)在37℃和5% CO2条件下温育48小时。存活率是通过使用细胞滴度格洛荧光素酶试剂或活细胞计数器测定ATP水平。数值转换为相比对照的抑制百分率, IC50曲线使用Prism的GraphPad数据的非线性回归分析拟合。 | |||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | cleaved PARP / cleaved caspase3 p-JAK2 / p-AKT / p-MAPK / Bcl-xl / MCL-1 c-Myc / c-Jun / Cyclin B / Cyclin D / Bcl-2 / HIF-1α p-STAT3 |

|
29849942 | |
| Growth inhibition assay | Cell viability Cell apoptosis Cell proliferation |
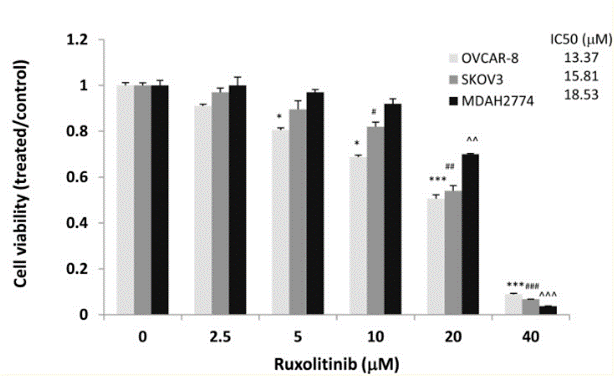
|
29849942 | |
| Immunofluorescence | α-tubulin |
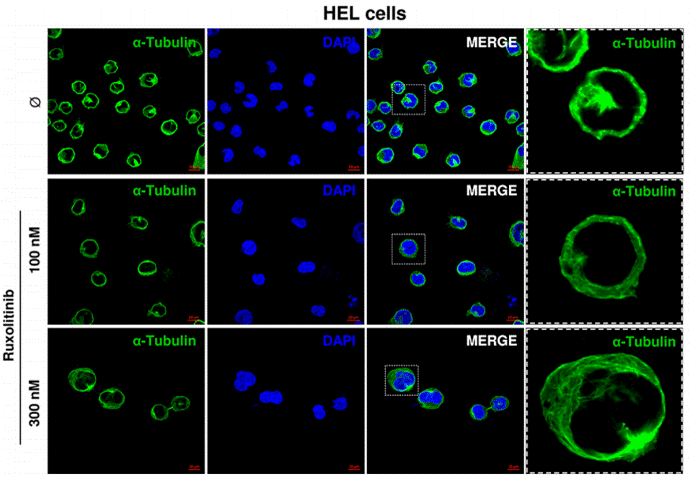
|
26356819 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | INCB018424(180 mg/kg,口服,每日两次)导致JAK2V617F驱动的小鼠模型的生存率在处理22天后大于90%。在JAK2V617F驱动的小鼠模型中,INCB018424(180 mg/kg,口服,每日两次)显着降低脾脏肿大和炎症因子的循环水平,并优先消灭肿瘤细胞,造成显著延长的生存期,无骨髓抑制或免疫抑制作用。[1] 在骨髓纤维化的双盲试验中,Ruxolitinib组的主要终点达到41.9%,安慰剂组则为0.7%。 Ruxolitinib导致脾体积持续减少和总症状得分提高50%或更多。[2] 在Ruxolitinib(15 mg,每天两次)组内,共28%骨髓纤维化患者至48周时脾脏体积减少至少35%,而接受最好的治疗组的比例为0%。Ruxolitinib致使脾脏长度减少了56%,而接受最好的治疗组却增加了4%。Ruxolitinib组患者的生活质量得到提高和骨髓纤维化相关症状减少。[3] | |
|---|---|---|
| 动物实验 | Animal Models | JAK2V617F驱动的小鼠模型 |
| Dosages | 180 mg/kg | |
| Administration | 口服 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06397313 | Not yet recruiting | Myelofibrosis |
Ryvu Therapeutics SA |
September 2024 | Phase 2 |
| NCT06388564 | Not yet recruiting | Chronic Graft-versus-host-disease |
Incyte Corporation |
July 8 2024 | Phase 2 |
| NCT06251102 | Not yet recruiting | Polycythemia Vera |
Gruppo Italiano Malattie EMatologiche dell''Adulto |
July 2024 | -- |
| NCT06343792 | Not yet recruiting | Steroid Refractory GVHD |
ReAlta Life Sciences Inc. |
May 2024 | Phase 2 |
|
化学信息&溶解度
| 分子量 | 306.37 | 分子式 | C17H18N6 |
| CAS号 | 941678-49-5 | SDF | Download Ruxolitinib (INCB18424) SDF |
| Smiles | C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3 | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 300 mg/mL ( (979.2 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 12 mg/mL (39.16 mM) Water : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项
常见问题及建议解决方法
问题 1:
What is the difference between S2902 and S1378 which seem to have same structure formula according to the product information?
回答:
These two chemicals are the two different chiral forms of this compound. S2902 S-Ruxolitinib is the S form and S1378 Ruxolitinib is the D form. One of the carbon atoms in it is asymmetric, making the two molecules mirror images of each other. The biological activities of these two molecules can be very different because of the confirmation differences.
问题 2:
How about the half-life of this compound? How long is the duration of its inhibitory effect on JAK-STAT signaling?
回答:
According to previous study, the half-life of this compound in body is about 2~3 hours. Generally, it is longer in vitro culture medium than in vivo. It was also used for 24 hours in paper. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=24711661.